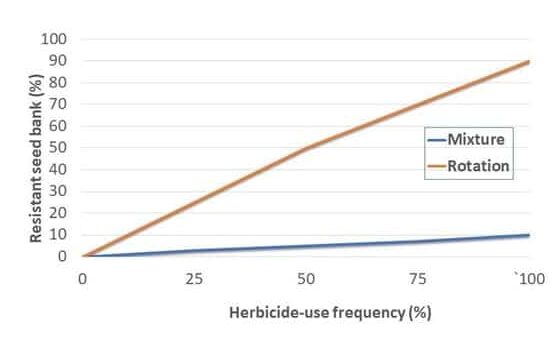
Finding and confirming herbicide-resistant weeds can be a real slog. It takes months, sometimes years, of field and lab work to confirm, replicate and publish the findings.
What if all you needed instead was a hole punch, a drone camera and some LED lights?
That’s exactly the user-friendly, rapid-test system that Dr. Eric Jones came up with during his graduate work at North Carolina State University. Jones proved this simple set-up can reliably detect glufosinate-resistant plants just 48 hours after exposure to the herbicide, in a paper published in the journal Weed Science in December 2022.
The experiment was so successful that Jones and the NC State team, led by weed scientists Wesley Everman and Ramon Leon, are now fine-tuning the test protocol on glufosinate-resistant Palmer amaranth and PPO-resistant redroot pigweed.
“What I would love to see is this becoming a quick tool that a farmer could use themselves, on their own workbench, in their shop, with a little light – rather than having to go out and flag down Extension every time they have a weed escape.”
Dr. Eric Jones, SDSU
The work is still in the “proof-of-concept” phase, but Jones, now an Extension weed specialist at South Dakota State University, has big dreams for it.
“What I would love to see is this becoming a quick tool that a farmer could use themselves, on their own workbench, in their shop, with a little light – rather than having to go out and flag down Extension every time they have a weed escape,” he says.
How Scientists Test for Resistance Now
Scientists currently rely heavily on traditional herbicide dose-response tests to identify herbicide-resistance. This means collecting seeds from a suspect plant, and then cleaning, chilling, and germinating them. Once grown, they are sprayed with varying doses of a herbicide to see when they do or do not die.
Those steps alone can take anywhere from three to six months – and then you need to replicate them.
“It’s the most tried-and-true way to confirm herbicide resistance,” as Jones notes. But by the time the replications are done, the results analyzed, and the message delivered to the agricultural community, that resistant population may already be entrenched in and beyond the field where it was found.
There are faster tests, such as molecular assays, which examine plant DNA for mutations, and chlorophyll fluorescence (CF) tests, which measure chlorophyll concentrations after a herbicide application. But they require expensive, highly specific equipment, Jones explains.
“We weren’t set up to do that,” he says of his Extension team at NC State. “We’re not a genomics research lab. We just wanted to create a tool that gives people the information they need somewhat rapidly, with low-tech equipment.”
It All Comes Down to Chlorophyll
Jones was initially inspired by CF tests, particularly work by Dr. Chenxi Wu, which used changes in the chlorophyll – the green pigment found in plant tissue – to identify plants resistant to dicamba, glyphosate and fomesafen (Flexstar, Reflex).
Armed with this example, Jones set out to create a similar test that wouldn’t require chlorophyll-measuring machines, which cost tens of thousands of dollars.
Instead he turned to the work underway at NC State using drone-mounted cameras to examine spectral reflectance – the way light reflects off a leaf – to identify weeds tucked within a soybean canopy.
“Essentially, there are different levels of green,” Jones explains. “The spectrum of light reflected back from a dark green and light green plant are different.”
If the drone cameras could detect different light spectrums from a Palmer amaranth weed growing alongside a soybean plant, surely they could also see the difference between a susceptible plant injured by glufosinate (Liberty) and a resistant plant that weathered the herbicide just fine, Jones realized.
How It Works
Glufosinate is a contact herbicide that can create symptoms on a susceptible plant in just 12 to 24 hours under ideal spraying conditions. The chemical destroys cell membranes, causing chlorophyll to leak out, essentially creating a leaf burn. “Pigments like chlorophyll are like a type of sunscreen – without them, plant tissue gets burnt,” Jones explains.
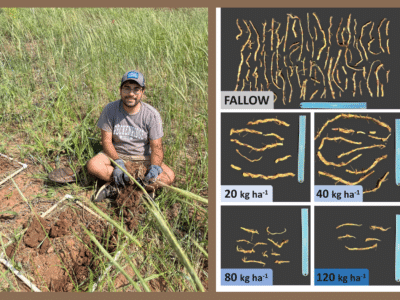
Jones and his team settled on glufosinate-resistant soybean and cotton varieties (LibertyLink) to test his system, because at the time, glufosinate-resistant weeds weren’t easily found. He paired the LibertyLink varieties with non-LibertyLink soybean and cotton plants, which would be susceptible to the glufosinate applications.
From there, the process was pretty simple. “If you can work a hole puncher, you can get this kind of assay set up,” Jones says. He punched holes in the LibertyLink and non-GMO plants, producing tiny leaf disks.

The disks were then submerged in water, with 10 varying rates of glufosinate concentrations, ranging from zero all the way up to 10 milli-molar of glufosinate per liter of water – a “pretty stout concentration,” as Jones puts it.
To mimic the sunshine that a glufosinate application needs to work, the submerged leaf disks were then immediately stowed away inside a light box, equipped with an LED light.
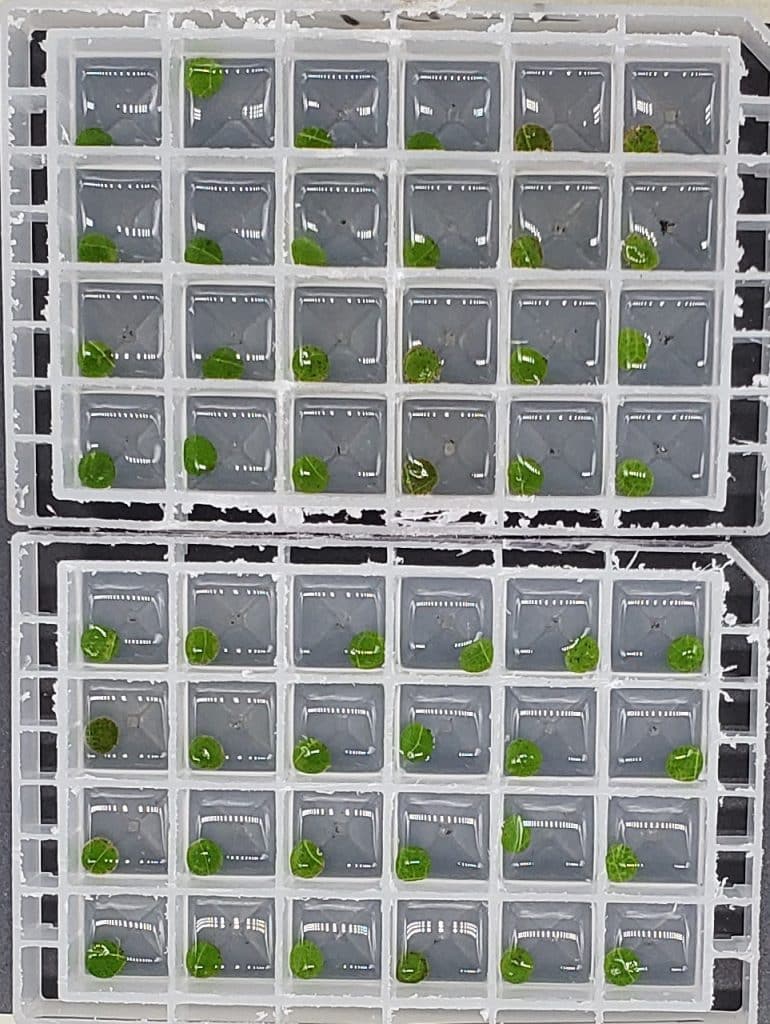

Around 48 hours, the change between the resistant and non-resistant leaf disks became clear.
Jones used a drone multispectral sensor, which could capture several different wavelengths of light, including RGB (red, green and blue) like a digital camera captures, as well as red edge and near-infrared light.

The pictures the sensor snapped were then fed into a simple software program, which pulled out all the different wavelengths captured by the LED light – red, green, blue and red edge. The software program then assigned numeric values to the different wavelengths in each leaf disk to see if there were any measurable differences among them.
It was a success: “We found that at the highest concentration, there was a big separation between the susceptible and resistant leaf disks,” Jones said.
Just like our eyes, the camera could see and measure the difference in a leaf injured by glufosinate, he concluded. But unlike our eyes, the test gave concrete, reliable numbers that could be rapidly compared and assessed. “It just takes the subjectivity out, by giving you hard numbers instead of just guessing with your eyes, which is tough to do when you are out walking a field,” Jones explains.

The researchers are now fine-tuning the necessary concentrations of herbicide to run a similar set-up with glufosinate-resistant Palmer amaranth and PPO-resistant redroot pigweed. They hope the work will lead to rapid test protocols that will take the guesswork out of finding herbicide-resistance early on in the field.
“My hope is that, after pulling problem weeds from the field, within a week you’d have actual numbers and graphs on each sample’s response,” Jones says.
For more information on herbicide resistance and how it is spread, see this GROW webpage.
Article by Emily Unglesbee, GROW. Photos by Eric Jones, South Dakota State University .