Amaranthus retroflexus L. and Amaranthus hybridus L.
Also known as:
A. retroflexus: redroot pigweed, rough pigweed, redroot amaranth, and careless weed
A. hybridus: smooth pigweed, hybrid amaranth, green pigweed, and slim pigweed

Biology
Redroot pigweed and smooth pigweed are summer annual weeds that look very similar and can be difficult to distinguish. They share most of the same characteristics that make them successful weeds. Their prolonged germination period, rapid growth rate, and prolific seed production make them major concerns for crop producers.
Due to having a C4 photosynthetic pathway, these weeds are efficient in photosynthesis and carbon dioxide fixation — that means they need less water to grow and reproduce than other weed species. They can reduce crop yield and quality and serve as hosts for crop pathogens and insect pests. Beyond the crop world, they can be toxic to livestock and produce pollen that causes allergic reactions in humans.
Redroot pigweed and smooth pigweed rely on seed dispersal for spread. They are both monoecious (male and female flowers are on the same plant) and can produce over 250,000 seeds per plant under favorable conditions. Redroot pigweed will occasionally hybridize with other Amaranthus species, and smooth pigweed is known to hybridize with common waterhemp and produce fertile new plant generations. At maturity, the stems of these weeds are 3 to 6 feet tall, branched, and have indeterminate growth habits. They respond to shade by growing taller and increasing the amount of leaf area to capture light more efficiently. Flower clusters are typically found at terminal panicles (the flowering structure at the end of branches) but can also be found at the leaf axils (where a leaf sprouts from the stem).
The seeds are dark, reddish brown, lens-shaped and around 1 millimeter in diameter. Redroot and smooth pigweeds can grow in a wide range of soil types, but redroot pigweed is less common in acidic soils. Some research suggests the lower soil pH in the southeastern United States limits the presence of redroot pigweed in that area. Both pigweeds emerge from the uppermost soil layer; their germination rates drop significantly when the seeds are buried an inch or deeper in the soil. As a result, they can be very problematic in no-till crop production systems. In addition to crop production fields, they are commonly found in gardens, waste places, roadsides, and other disturbed habitats.
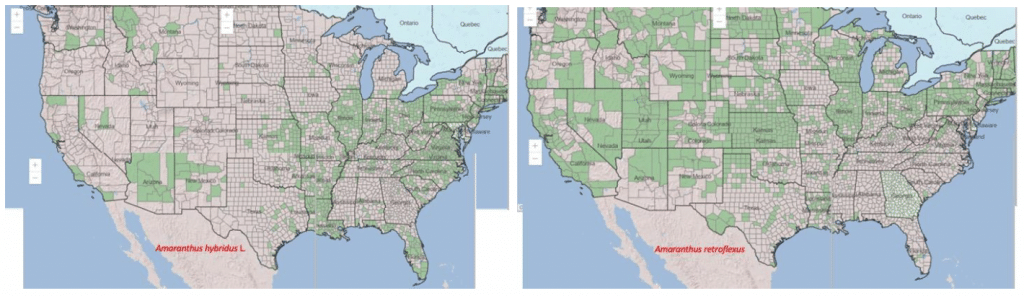
Where are redroot and smooth pigweeds a problem?
Redroot pigweed is native to eastern and central North America, while smooth pigweed is native to eastern North America, Mexico, and Central and South America. Smooth pigweed is more prevalent in regions with higher rainfall or along streams and riverbanks.
Both species are currently found in the United States mainland. However, due to its shorter life cycle, redroot pigweed extends further north than smooth pigweed and can be found in Canada and Alaska. Smooth pigweed is better adapted to warmer climates and lower altitudes.
What is the emergence pattern of redroot and smooth pigweeds?
The minimum germination temperature for redroot pigweed is 41 degrees Fahrenheit, but the weed’s preferred temperature range for maximum germination is from 77 to 104 degrees. Optimum germination of smooth pigweed seeds is found between 90 and 93 degrees. They germinate better in light than in darkness, so buried seeds often germinate following soil disturbance.
Redroot and smooth pigweeds are considered later-emerging weeds with a prolonged emergence period. Depending on location and environment, these weeds can start emerging in late spring to early summer and continue to germinate throughout the summer if adequate soil moisture is available. This extended emergence window allows late-emerging plants to avoid common postemergence weed control tactics. These weed escapes may not impact crop yields of the current year but will increase the weed seedbank for subsequent years.
What is the lifecycle of redroot and smooth pigweeds?
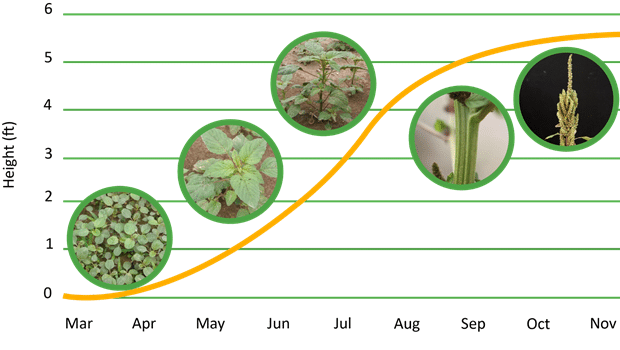
Redroot and smooth pigweed seedlings emerge over a prolonged period with peak emergence from late spring to summer, depending on the location. Plants do not tolerate shade and complete their life cycle before the onset of frost. As daylight hours decrease later in the season, the plants’ reproductive development speeds up. Generally smooth pigweed has a longer life cycle and matures later than redroot pigweed. Mature plants produce a large number of seeds that are dormant and do not germinate immediately in a North American climate.

How do redroot and smooth pigweeds spread?
Redroot and smooth pigweed seeds are small with no special adaptations for dispersal, and as a result, they often fall near the mother plant. However, other methods of movement such as farm machinery, water, birds, animals, and the spreading of manure and compost can disperse them farther away. Their seeds can float easily, and they are able to move by rain runoff, surface irrigation, and water courses. Redroot pigweed was among the most common weed seeds found in surface-irrigation canals in western Nebraska. Viable seeds can also be dispersed after ingestion and elimination by birds and mammals such as sheep and cattle.

How many seeds can redroot and smooth pigweeds produce and how long can they survive?
Both species are prolific seed producers and can produce more than 250,000 seeds per plant under favorable growing conditions. Under near-perfect conditions, without crop competition, redroot pigweed plants have produced over one million seeds per plant. More commonly, pigweed plants growing in competition with a crop can produce 10,000 to 40,000 seeds.
Shade, moisture stress, and delayed emergence can reduce seed production considerably. Both species retain most of their seeds until the average soybean harvest timing in the fall. A nationwide U.S. seed rain study showed that smooth pigweed retained higher amounts (95%) than redroot pigweed (75%) four weeks after full-season soybean maturity.
At maturity, seeds are dormant but may germinate under high temperature and light conditions. Seed survival varies with burial depth, temperature, moisture, and dormancy, but most studies report that buried seeds of redroot pigweed can remain viable for at least 6 years. In a study in Colorado, redroot pigweed seed reserves in the soil were reduced by 95% in four years when the weeds were controlled, and no plants were permitted to produce seeds. When excellent weed control was not achieved, the number of seeds in the soil quickly rebounded.

What other biological weaknesses do redroot and smooth pigweed have that can be targeted with management techniques?
Both species have small seeds, and most of the seeds are light sensitive for germination; only seeds near the surface of the soil are able to emerge due to limited carbohydrate reserves. The need to be close to the soil surface is an attribute that can be targeted with mechanical and cultural weed control. The species’ high seed retention up to crop harvest makes it suitable for mechanical removal or harvest weed seed control technologies. (See the management section below).
Herbicide Resistance
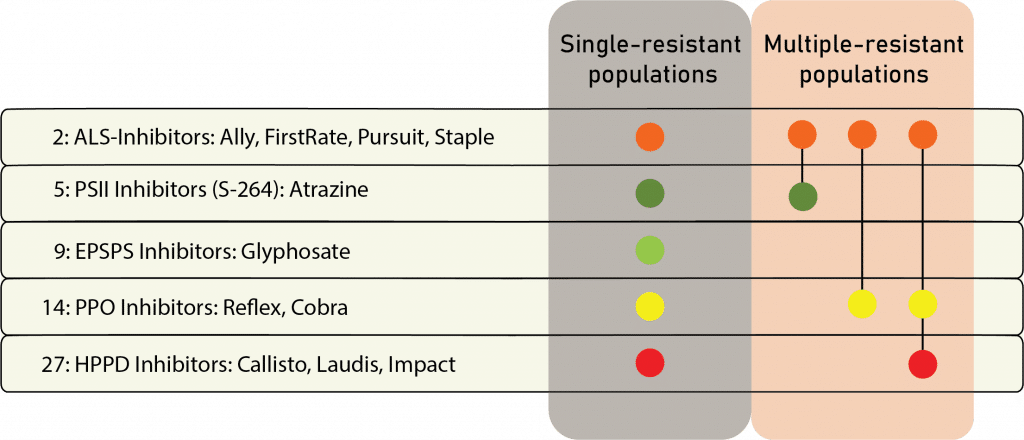
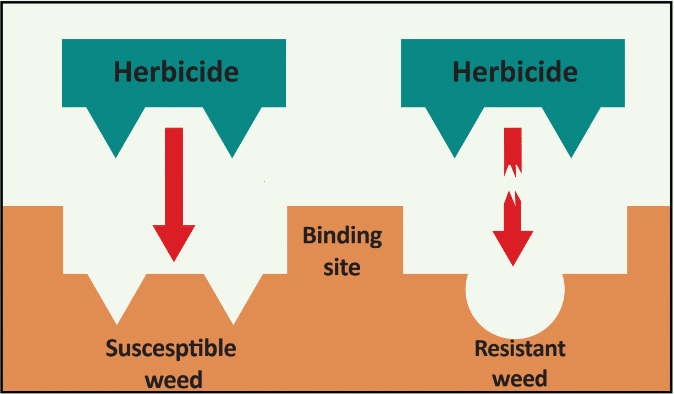
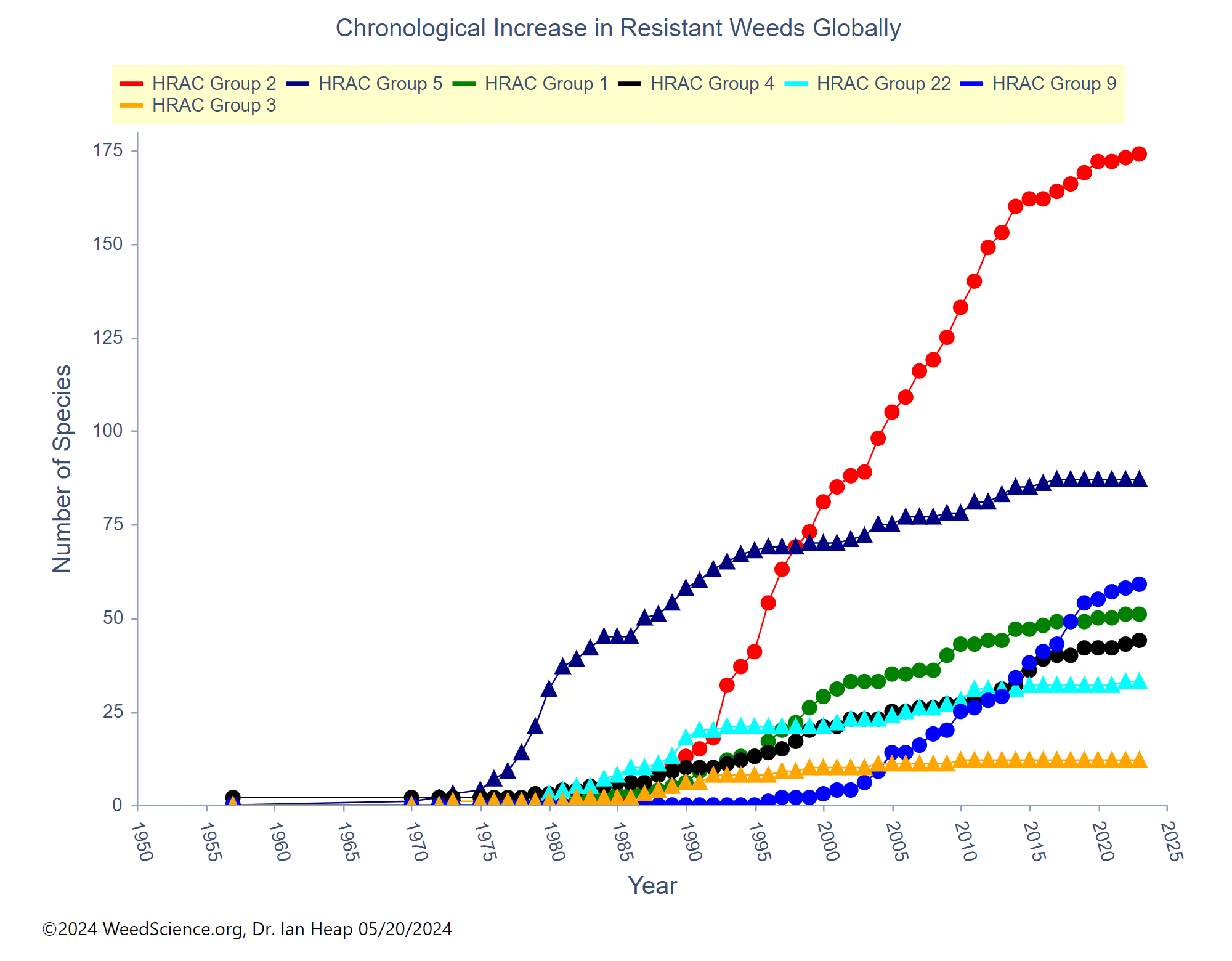
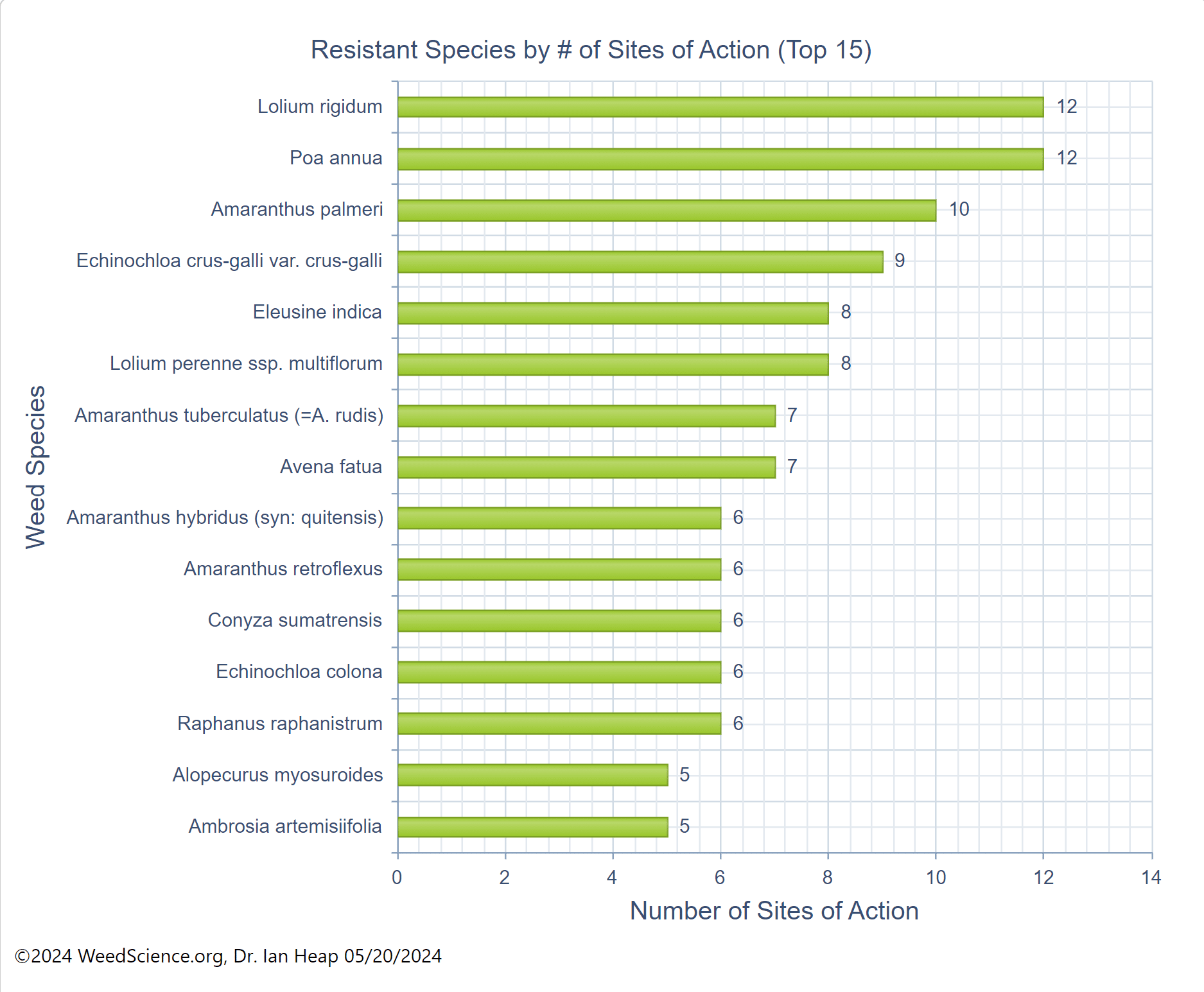
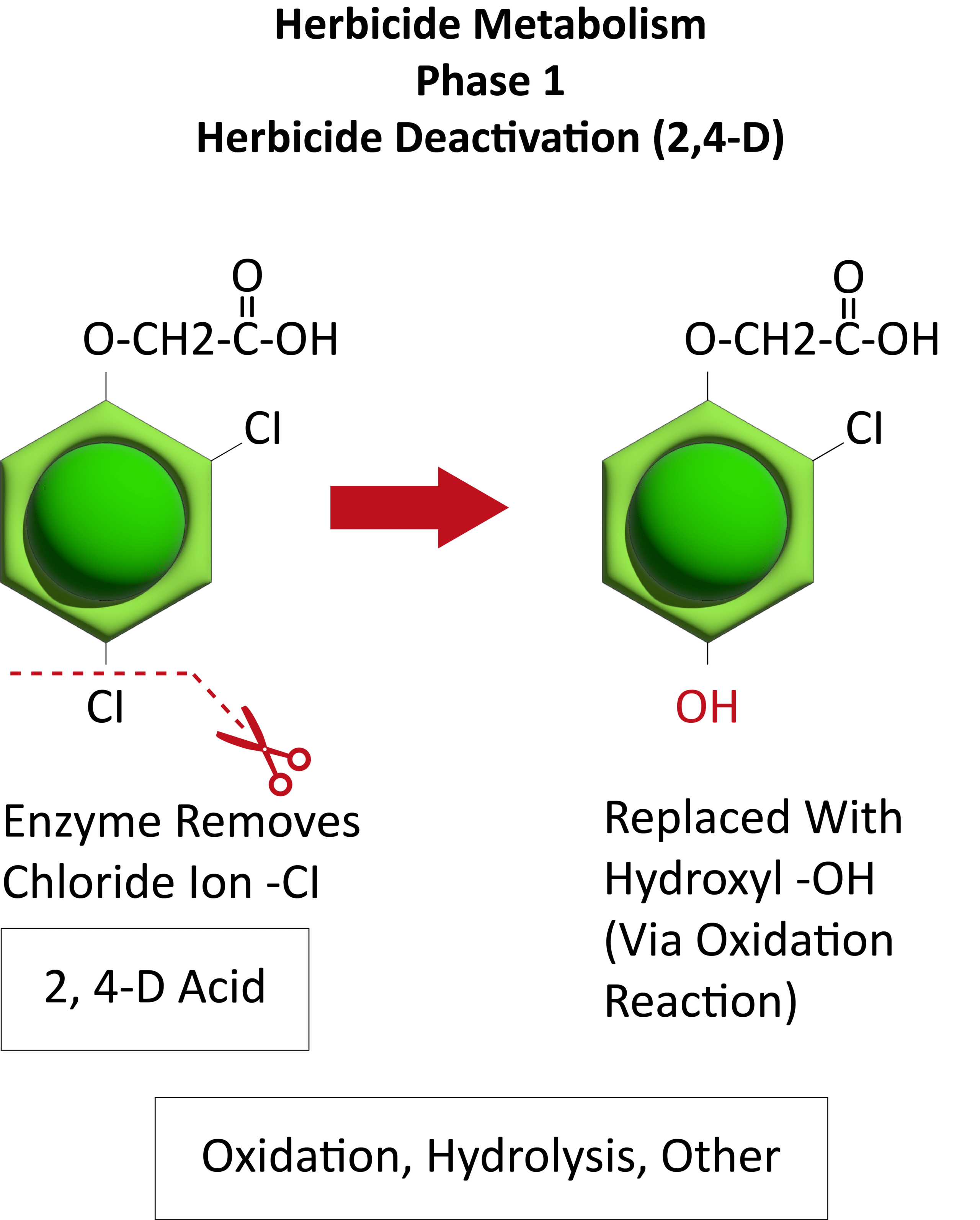
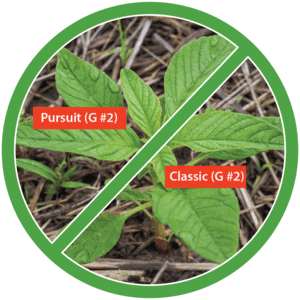
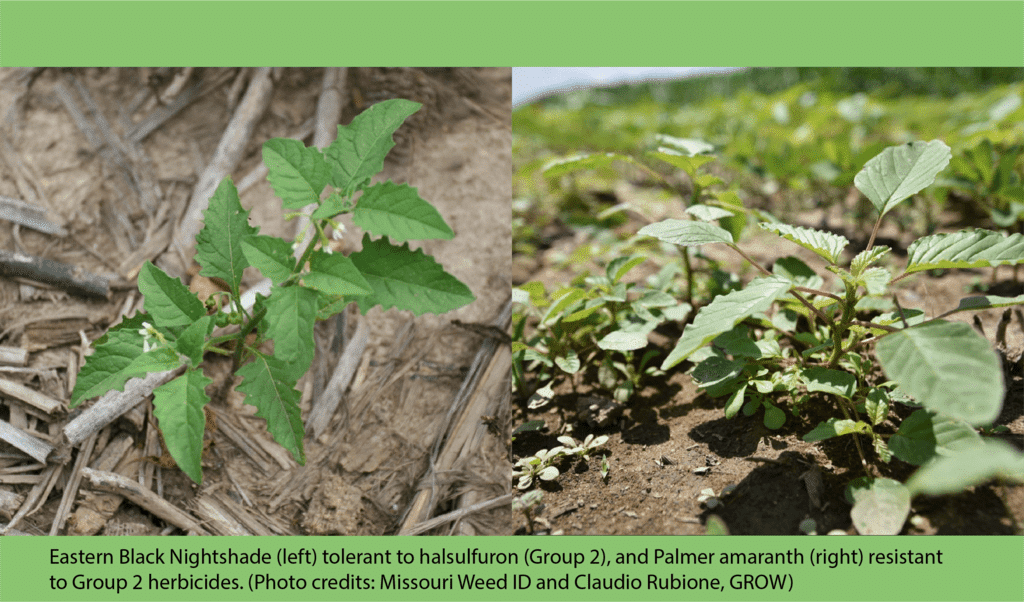
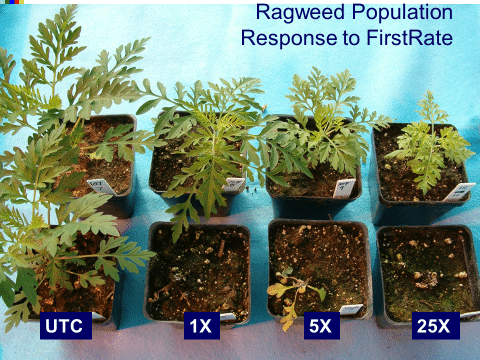
The first report of triazine resistance worldwide was a smooth pigweed population in the US in 1972. Redroot pigweed resistance to Group 2 herbicides was first reported in North America in 1995. Resistant populations of both species to Group 2 and Group 5 herbicides are now widespread in North America. In 2022, a redroot pigweed population from North Carolina was found to be resistant to ALS-inhibiting herbicides (Group 2), PPO-inhibiting herbicides (Group 14), and HPPD-inhibiting herbicides (Group 27). Glyphosate (Group 9) and auxin-resistance (Group 4) have been reported in smooth pigweed in other parts of the world, but not yet in the US. Contact your local extension office for details about resistance in your area and management options.
*Herbicide names listed are representative products that contain specific active ingredients. Last updated on: 02-11-2022
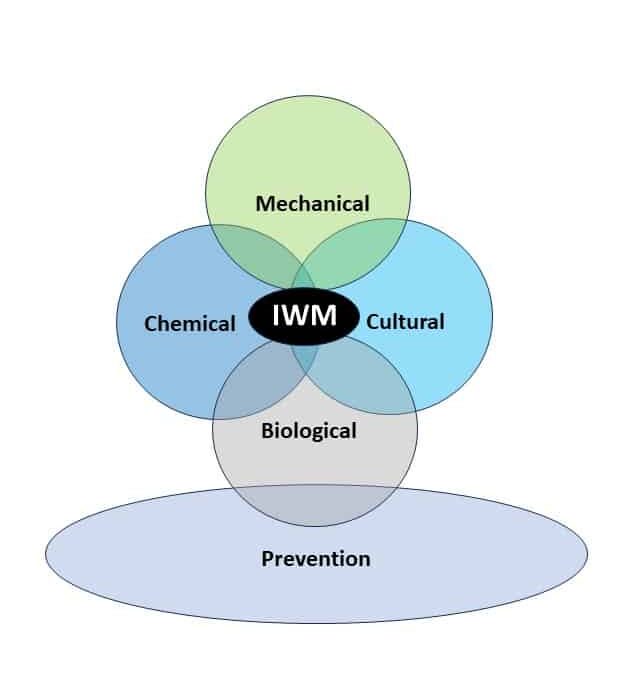
Integrated Weed Management Strategies for Control
Cultural practices that are essential for good control of these pigweed species include crop rotation and maximizing crop competitiveness. Rotations involving perennial forage crops such as alfalfa with repeated cuttings can help to minimize pigweed seed production. Fall-seeded small grain crops are established well before these pigweeds start to emerge and can help smother the weeds during pigweed’s spring emergence period. And since smooth and redroot pigweed don’t tolerate shade, cropping practices that quickly develop a crop canopy to shade the soil will reduce weed competition. For instance, soybean canopies close seven to 15 days earlier at 15-inch row spacing compared to 30-inch row spacing, improving early season crop competitiveness.
Scouting and proper identification of smooth and redroot pigweed are important. Smooth and redroot pigweed can look similar to other pigweed species and might be found together with other much more aggressive species of the same genus such as Palmer amaranth or waterhemp. Since all of the amaranth species are known to evolve herbicide resistance, preventing seed production and replenishing the weed seedbank is important irrespective of economic thresholds. Scouting can determine if management tactics were successful and detect any potential new resistance cases.
Cover crops can help with pigweed control through reduced emergence and growth. Cover crop species that produce high amounts of biomass that persists (such as cereal cover crops) are the most suitable cover crop for pigweed management. Legume cover crops are less effective in suppressing pigweeds, because they add nitrogen to the soil, and their residue dissipates quickly.
To achieve maximum biomass, early establishment of the cover crop and/or delayed spring termination is essential. In northern climes, interseeding into standing cash crops can help accomplish this. Delayed termination timing is also important because it allows for maximum development of plant stems which do not break down quickly. There is some concern with terminating too close to crop planting due to the potential for “green bridges” for insect pests and disease and avoiding nutrient tie-up, particularly before corn. But some growers are finding success with terminating cereal rye at or after planting (planting green), particularly in soybean.
Cereal rye can be terminated either using a roller crimper (mostly for organic growers) or through a herbicide application, such as glyphosate. For optimum pigweed control, cover crops should be integrated with preemergence and postemergence herbicide applications. Be mindful of potential herbicide carryover injury to cover crops from residual herbicides and potential antagonism when tank mixing soil-applied herbicides with herbicides for cover crop termination.
Prevention is an important but often overlooked management strategy. Using weed-free, certified seed is critical. Cleaning clothing, shoes, and agricultural equipment, including tractors and combines, to prevent spread is important. If mature pigweed plants are present at harvest, plan the harvest schedule to limit spread within a field by harvesting weed-free areas first and thoroughly cleaning the combine before moving to other fields.
Mechanical weed control through tillage, inter-row cultivation, mowing, flaming and harvest weed seed control (HWSC) can be an important addition to the pigweed management toolbox. Due to their small seeds, limited reserves in the seeds, and fragile taproots, pigweeds are more susceptible than other weed species to “blind cultivation” – the practice of cultivating a field with no attention to field rows, with implements such as rotary hoes or spring-tine cultivators. Blind cultivation should be done before seedlings progress beyond the cotyledon stage.
Shallow inter-row cultivation can be a useful tool, especially in organic crop production and should be used to target small seedlings under three inches tall. Cultivation may be less effective on mature weeds, needs to be repeated and should not be adopted as a standalone pigweed management technique. Shallow tillage can increase pigweed emergence, but in heavily infested fields, strategically timed deep tillage can bury the seed beyond the depth of emergence, reducing emergence from the surface soil. However, frequent deep tillage can bring seeds back to the surface, so moldboard plowing management should only occur once every four or more years.
Stale-seedbed – the practice of allowing weed seeds just below the soil surface to germinate in order to terminate them – is effective on these species. Most of the plants will emerge by early summer, but emergence at a much slower rate will continue into late summer. A two-week interval between tillage passes will help stimulate emergence with the second pass destroying most of the seedlings.
Mowing smooth and redroot pigweed along roadsides or field edges can decrease seed production, but will not kill plants. Multiple mowings within a season are needed to reduce pigweed seed production. Mowing plants with mature seeds may result in spreading the infestation and should be avoided.
Harvest weed seed control (HWSC) involves processing the crop chaff exiting a combine during harvest to minimize weed seeds returning to the field. HWSC is most successful on weeds that retain their seeds up to crop maturity and during crop harvest. High seed retention in these species (95% for smooth pigweed and 80% for redroot pigweed) suggests that the technology is suitable to reduce next-year pigweed emergence and herbicide selection pressure in the field. These techniques are most effective for soybean and small grain production systems where a combine cutter bar collects all aboveground crop material during harvest. There are various HWSC systems currently in use around the world. Among these, chaff lining (which concentrates chaff into rows) and impact mills (which pulverize chaff to make weeds seeds nonviable) are under investigation by various North American institutions. Adopting this technology could help minimize pigweed seed deposition and future populations.
Chemical control remains an important tool for pigweed management. It is important to start clean (no emerged weeds at planting) either through tillage or through burndown herbicide applications. Glyphosate (Roundup), glufosinate (Liberty), dicamba (Banvel, Clarity), 2,4-D (2,4-D), and paraquat (Gramoxone) are effective for burndown control of both species. A preemergence residual herbicide application followed by at least one postemergence application is required to achieve early and late-season pigweed control. When applied as preemergence herbicides, various premixes of the following herbicides can provide effective control for three to four weeks: Group 3 [pendimethalin (Prowl)], Group 5 [metribuzin (Sencor), atrazine (Atrazine)], Group 7 [linuron (Lorox)], Group 14 [flumioxazin (Valor), sulfentrazone (Spartan), saflufenacil (Sharpen)], Group 15 [acetochlor (Warrant, Harness), dimethenamid (Outlook), metolachlor (Dual), pyroxasulfone (Zidua)], and Group 27 [mesotrione (Callisto), isoxaflutole (Balance)].
Postemergence herbicides should be applied when pigweeds are less than four inches in height. If the population is susceptible to them, ALS inhibitors and atrazine provide effective control. Resistance to PPO inhibitors (acifluorfen, fomesafen, and lactofen) and HPPD inhibitors (Callisto, Armezon, Laudis) has been reported in North Carolina. Herbicides from Group 4 [(2,4-D (2,4-D), dicamba (Banvel, Clarity)], Group 9 [glyphosate (Roundup)], Group 10 [glufosinate (Liberty)], Group 14 [fomesafen (Reflex), lactofen (Cobra)], and Group 27 [mesotrione (Callisto)] can be applied in appropriate crops and crop traits for effective control. For optimum pigweed control with PPO herbicides and glufosinate (Liberty), target weeds under four inches tall with good coverage (15 GPA of water or more and medium droplets). Given their wide emergence period, tank-mixing residual herbicides at the time of postemergence herbicide application can minimize pigweed seed production. Group 15 herbicides [acetochlor (Warrant), dimethenamid (Outlook), pyroxasulfone (Zidua), S-metolachlor (Dual II Magnum)] are effective and suitable for postemergence tank-mixes in many summer crops. Refer to your local Extension’s weed management guide for effective options.
Biological control of smooth and redroot pigweed relies on ecological processes since no bio-control commercial products are available. There are leaf-feeding insects that attack smooth pigweed but the damage caused seldom kills the plant or stops seed production. The amaranth flea beetle (Disonycha glabrata) consumes foliage and Coleophora lineapulvella caterpillars feed on Amaranthus seeds before dispersal. Some fungal pathogens like Myrothecium verrucaria infect Amaranthus plants and may be serving as population checks. Seeds on or near the soil surface or within chaff lines are often consumed by rodents and invertebrates such as ground beetles (Carabidae), crickets (Gryllidae), and mice. Ground cover can increase the density of these seed predators. Wildlife (i.e. ducks and geese) will also consume these species’ seeds, however, if seeds remain viable through the animal’s digestive system, the seeds can be dispersed in new areas.
Similar Weed Species
There are over forty-five species in the Amaranthus genus in the United States. Redroot pigweed (Amaranthus retroflexus) and smooth pigweed (Amaranthus hybridus) are closely related to other common and problematic pigweed species, including waterhemp (Amaranthus tuberculatus), Palmer amaranth (Amaranthus palmeri), Powell amaranth (Amaranthus powellii), and spiny amaranth (Amaranthus spinosus). It is important to distinguish redroot and smooth pigweed from waterhemp and Palmer amaranth due to differences in response to management. Currently, herbicide resistance in these species is not as problematic as waterhemp and Palmer amaranth, so more herbicide choices are available for both the species. The table below compares the characteristics of the most common pigweeds to infest summer grain crops in North America.
| Characteristic | Palmer Amaranth | Waterhemp | Spiny Amaranth | Redroot Pigweed* | Smooth Pigweed* | Powell Amaranth |
| Stem Hairs | No | No | No | Yes | Yes | Yes |
| Leaf Shape | Diamond-shaped | Long, narrow | Egg-Shaped | Egg-Shaped | Egg-Shaped | Egg-Shaped |
| Petiole Length | Long, often as long as leaf blade | Medium, half the length of the leaf blade | Medium, 1/2 inch spines where petioles attach to stem | Short | Short | Short |
| Separate Male/Female Plants | Yes | Yes | No | No | No | No |
| Seedhead | Prickly, long, thick | Smooth, long, slender | Male flowers at top and female at middle | Prickly, short, stout | Slightly prickly, long, slender | Prickly, long, thick |
| Watermarks | Occasionally | No | Often | No | No | No |
*Separating redroot pigweed from smooth pigweed relies on the length at maturity of sepals/tepals (the protective structures that enclose a plant’s flower and ultimately, its seed): Sepals of redroot pigweed are about twice as long as the seed and often curved outward; Sepals of smooth pigweed are the same length as seed.
Authors
Navjot Singh
Claudio Rubione
Debalin Sarangi
Editors
Victoria Ackroyd
Kreshnik Bejleri
Michael Flessner
Eugene Law
Emily Unglesbee
Mark VanGessel
Reviewed By
William Curran
Gallery









Resources
Adjesiwor A, Prather T, Felix J, Morishita D (2021) Pigweeds: Current and Emerging Weed Threats in the Pacific Northwest. Pacific Northwest Extension Publishing.
Heap I (2021) The International Herbicide-Resistant Weed Database www.weedscience.org Accessed: April 6, 2021. Licht M (2018) Consider 15-inch Row Spacing in Soybean https://crops.extension.iastate.edu/cropnews/2018/02/consider-15-inch-row-spacing-soybean
LeRoy G, Holm I, Pancho J, Herberger J (1977) The World’s worst weeds: distribution and biology. East-West Center, University Press of Hawaii, Honolulu, Hawaii, USA. pp 114-118
Liebman M, Mohler C, Staver C (2001) Ecological management of agricultural weeds. Pages 65, 73, 158, 222, 225, 247, 296, 333, 202 in Liebman, M., C.L. Mohler and C.P. Staver. Ecological Management of Agricultural Weeds. New York: Cambridge University Press.
Mohler C, Teasdale J, DiTommaso A (2021) Pigweeds. Pages 338-343 in Mohler CL, Teasdale JR, DiTommaso A, eds. Manage Weeds On Your Farm: a guide to ecological strategies.
USDA-SARE Handbook Series 16 https://www.sare.org/resources/manage-weeds-on-your-farm/
Penn State Extension (2016) Herbicide recommendations for management of noxious pigweeds in corn, soybean, and forages- Penn State https://extension.psu.edu/herbicide-recommendations-for-noxious-pigweeds
Citations
Bensch C, Horak J, Peterson D (2003) Interference of redroot pigweed (Amaranthus retroflexus), Palmer amaranth (A. palmeri), and common waterhemp (A. rudis) in soybean. Weed Science 51:37–43 doi.org/10.1614/0043-1745(2003)051[0037:IORPAR]2.0.CO;2
Blackshaw R., Rode L (1991) Effect of ensiling and rumen digestion by cattle on weed seed viability. Weed Science 39:104-108 doi.org/10.1017/S0043174500057957
Bunchek J, Wallace J, Curran W, Mortensen D, VanGessel M, Scott B (2020) Alternative performance targets for integrating cover crops as a proactive herbicide-resistance management tool. Weed Science 68:534-544 doi.org/10.1017/wsc.2020.49
Burnside O, Wilson R, Weisberg S, Hubbard K (1996) Seed longevity of 41 weed species buried 17 years in eastern and western Nebraska. Weed Science 44:74-86 doi.org/10.1017/S0043174500093589
Costea M, Weaver S, Tardif F (2004) Biology of Canadian weeds 130. Amaranthus retroflexus L., A. powellii S. Watson and A. hybridus L. Canadian Journal of Plant Science 84:631–668 doi.org/10.4141/P02-183
DeSousa N, Griffiths J, Swanton C (2003) Predispersal seed predation of redroot pigweed (Amaranthus retroflexus) Weed Science 51:60-68 doi.org/10.1614/0043-1745(2003)051[0060:PSPORP]2.0.CO;2
Eghball B, Lesoing G (2000) Viability of weed weeds following manure windrow composting. Compost Science & Utilization 8:46-53 doi.org/10.1080/1065657X.2000.10701749
Egley G, Chandler J (1983) Longevity of weed seeds after 5.5 years in the Stoneville 50-year buried-seed study. Weed Science 31:264-270 doi.org/10.1017/S0043174500068958
Gfeller A, Herrera J, Tschuy F, Wirth J (2021) Explanations for Amaranthus retroflexus growth suppression by cover crops. Crop Protection 104:11-20 doi.org/10.1016/j.cropro.2017.10.006
Hoagland R, Teaster N, Boyette C. (2013) Bioherbicidal effects of Myrothecium verrucaria on glyphosate-resistant and -susceptible Palmer amaranth biotypes. Allelopathy journal 31:367-376 https://pubag.nal.usda.gov/download/60787/pdf
Khan A, Mobile A, Werth J, Chauhan B (2021) Effect of emergence time on growth and fecundity of redroot pigweed (Amaranthus retroflexus) and slender amaranth (Amaranthus viridis): emerging problem weeds in Australian summer crops. Weed Science 69:333-340 doi.org/10.1017/wsc.2021.9
Knezevic S, Horak M (1998) Influence of emergence time and density on redroot pigweed (Amaranthus retroflexus). Weed Science 46:665-672. doi.org/10.1017/S0043174500089694
Loux M, Dobbels A, Bradley K, Johnson W, Young B, Spaunhorst D, Steckel L (2017) Influence of cover crops on management of Amaranthus Species in glyphosate- and glufosinate-resistant soybeans. Weed Technology 31:487-495 doi.org/10.1017/wet.2017.30
Mclachlan S, Tollenaar M, Swanton C, Weise S (1993) Effect of corn-induced shading on dry matter accumulation, distribution, and architecture of redroot pigweed (Amaranthus retroflexus). Weed Science 41:568-573 doi.org/10.1017/S0043174500076335
Mohler C, Teasdale J (1993) Response of weed emergence to rate of Vicia villosa Roth and Secale cereale L. residue. Weed Research 33:487-499 https://doi.org/10.1111/j.1365-3180.1993.tb01965.x
Norris R (2007) Weed fecundity: Current status and future needs. Crop Protection 26:182-188 doi.org/10.1016/j.cropro.2005.07.013
Palhano M, Norsworthy J, Barber T (2018) Evaluation of chemical termination options for cover crops. Weed Technology 32:227-235 doi.org/10.1017/wet.2017.113
Rajcan I, AghaAlikhani M, Swanton C, Tollenaar M. (2002) Development of redroot pigweed is influenced by light spectral quality and quantity. Crop Science 42 doi.org/10.2135/cropsci2002.1930
Schwartz-Lazaro L, Norsworthy J, Walsh M, Bagavathiannan M (2017) Efficacy of integrated Harrington seed destructor on weeds of soybean and rice production systems in the southern United States. Crop Science 57:2812-2818 doi.org/10.2135/cropsci2017.03.0210
Schwartz-Lazaro L, Shergill L, Evans J, Bagavathiannan M, Beam S, Bish M, Mirsky S (2021) Seed-shattering phenology at soybean harvest of economically important weeds in multiple regions of the United States. Part 1: Broadleaf species. Weed Science 69:95-103 doi.org/10.1017/wsc.2020.80
Schwartz-Lazaro L, Shergill L, Evans J, Bagavathiannan M, Beam S, Bish M, Mirsky S. (2021) Seed-shattering phenology at soybean harvest of economically important weeds in multiple regions of the United States. Part 1: Broadleaf species. Weed Science, 69:95-103 doi.org/10.1017/wsc.2020.80
Sellers B, Smeda R, Johnson W, Kendig J, Ellersieck M (2003) Comparative growth of six Amaranthus species in Missouri. Weed Science 51:329-333 doi.org/10.1614/0043-1745(2003)051[0329:CGOSAS]2.0.CO;2
Van der Laat R, Owen M, Liebman M, Leon R (2015) Postdispersal weed seed predation and invertebrate activity density in three tillage regimes. Weed Science 63:828-838 doi.org/10.1614/WS-D-15-00030.1
Whalen D, Bish M, Young B, Hager A, Conley S, Reynolds D, Bradley K (2019) Evaluation of cover crop sensitivity to residual herbicides applied in the previous soybean [Glycine max (L.) Merr] crop. Weed Technology 33:312-320 doi.org/10.1017/wet.2019.10
Zhong Huang J, Shrestha A, Tollenaar M, Deen W, Rahimian H, Swanton C (2000) Effects of photoperiod on the phenological development of redroot pigweed (Amaranthus retroflexus L.) Canadian Journal of Plant Science 80:929-938 doi.org/10.4141/P99-134