Amaranthus tuberculatus (Moq.) Saur
(syn. A. rudis Sauer)
Also known as common waterhemp; tall waterhemp; pigweed; roughfruit amaranth

Biology
Waterhemp is a summer annual plant, relying upon yearly seed production for persistence. It is one of the few problematic dioecious weeds in US cropping systems, which means that male and female flowers occur on different plants. Dioecious species require the exchange of genetic information, primarily through wind-dispersed pollen flow, to produce seeds. This mandatory genetic exchange is thought to have allowed waterhemp to rapidly adapt to its environment and management, and has led to variation in form (morphology) within the species, such as differences in branching patterns, plant size, and stem, leaf, and flower color and shape, as well as widespread herbicide resistance.
Waterhemp grows rapidly, up to one inch per day under favorable conditions. Waterhemp is also highly competitive for resources such as nitrogen and soil moisture. When emerging at the soybean unifoliate stage, dense waterhemp populations (8 to 34 plants/ft2) reduced soybean yields 43% in Illinois, and 27 to 63% in Kansas. When competing with corn, yield losses ranged from 11 to 74%, depending upon soil moisture availability. The plant’s taproot can extend over 25 inches deep into the soil, and secondary roots may reach over six ft beyond the plant stem. Additionally, the plant produces allelopathic compounds (biochemicals) which may interfere with germination and growth of other plants.
Where is waterhemp a problem?
Waterhemp is native to the United States. This species often thrives in disturbed habitats, such as agricultural fields, and has historically been associated with waterways and floodplains. However, since the 1950’s waterhemp has increasingly become associated with agricultural fields and is now a problem for crop producers across a broad geographic range. Conservation tillage and the evolution of resistance to multiple herbicides have contributed to the plant’s adaptive success. Waterhemp has adapted to tolerate a wide range of environmental conditions, preferring well-drained, nutrient rich soils, which are also preferred for crop cultivation. The plant tolerates a soil pH range of 4.5 to 8 and temporary flooding but has little tolerance for salinity.
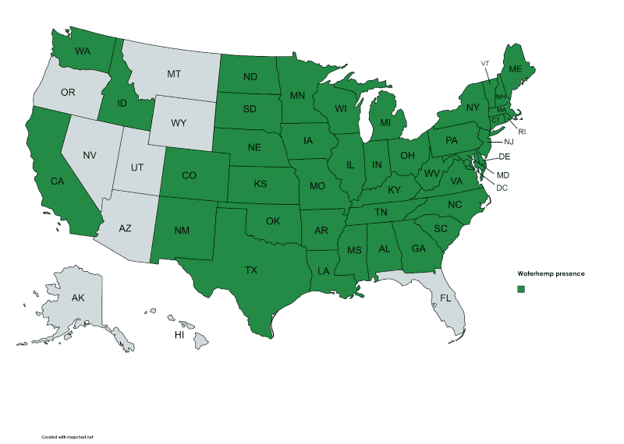
(Map Credit: USDA Plants Database) Amaranthus tuberculatus
What is the emergence pattern of waterhemp?
Waterhemp has a prolonged emergence pattern, compared to other annual weed species. Waterhemp may begin emerging in late-April or early-May and continue until late-summer in southern Illinois, depending upon rainfall patterns. Optimal temperatures for emergence are between 90 to 95 ℉, but minimum temperatures may depend on geographic origin and range from 50 to 65 ℉ across Iowa and Illinois, respectively.
What is the lifecycle of a waterhemp plant?
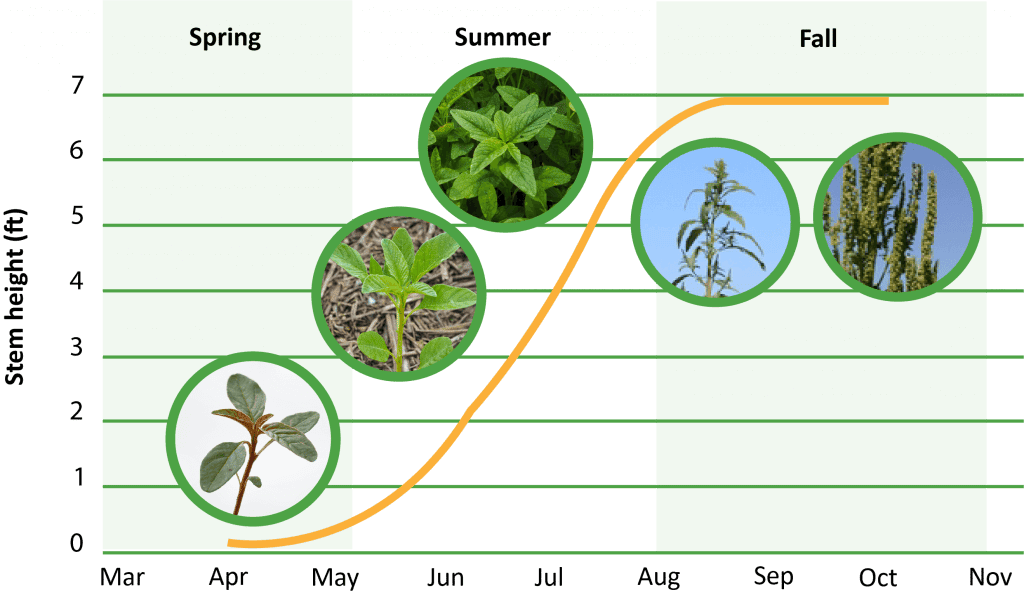
How does waterhemp spread?
New introductions into agricultural fields may be due to human activities, such as the movement of machinery. Seed may also be dispersed by water, birds and other animals, and to some degree wind. Seeds may also move via contaminated manure and compost. Waterfowl have been documented to disperse pigweed seeds including waterhemp, with an estimated 1800 mile range.
How many seeds can waterhemp produce and how long can those seeds survive?
Female plants may produce over 1 million seeds, if plants emerge early enough in the season and have plenty of resources for growth. Up to 80% of seeds produced are viable and may produce a persistent seedbank. In a 3-year study in Illinois, Tennessee, and Missouri, 4.3% of waterhemp seed on the surface of the soil remained viable, compared to 5.3% of seed that remained viable after the same period when buried at six inches. In Nebraska, 1 to 3% of seeds that were buried at eight inches were viable after 17 years.
What other biological weaknesses does waterhemp have that can be targeted with management techniques?
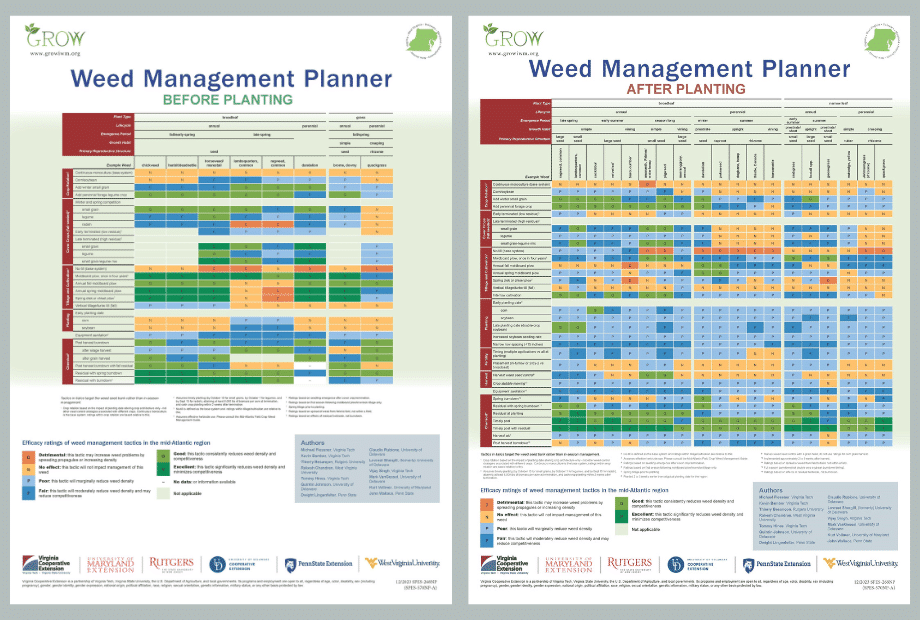
Before planting a crop, it is important to start with a weed-free seedbed through an effective practice such as the use of burndown herbicides or tillage. Waterhemp is easiest to control when in the seedling stage. Waterhemp seeds are small and must germinate near the surface of the soil. Practices that limit light exposure to the soil surface, such as cover crop residue, crop canopy closure, or targeted tillage, may reduce waterhemp populations. Management efforts should seek to reduce the number of waterhemp plants setting seed and contributing to the seedbank. With a relatively short-lived seedbank, eliminating seed production for 3 to 5 years will result in a dramatic reduction in population density. Managing the seedbank will exploit the weakest link in waterhemp’s life cycle. However, managing waterhemp for zero seed production requires additional management and expense. A combination of tactics in an integrated weed management approach is necessary.
Herbicide Resistance
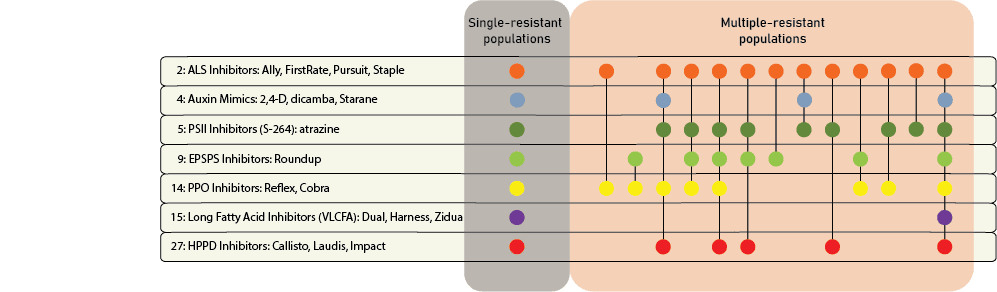
Contact your local extension office for details about resistance in your area and management options.
*Herbicide names listed are representative products that contain specific active ingredients. Last updated on: 4-01-2021
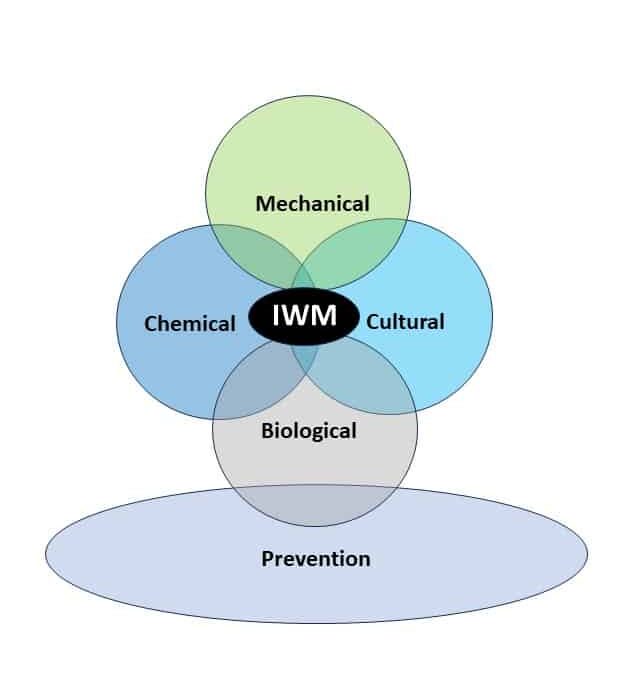
Integrated Weed Management Strategies for Control
Cultural practices that maximize crop competitiveness enhance overall weed control. Crop planting densities and row spacings should be optimized to encourage early closure of the crop canopy, which reduces the amount of light reaching the soil and subsequent weed emergence. Similarly, crop residue may suppress germination of annual weeds by 80% in a no-till system.
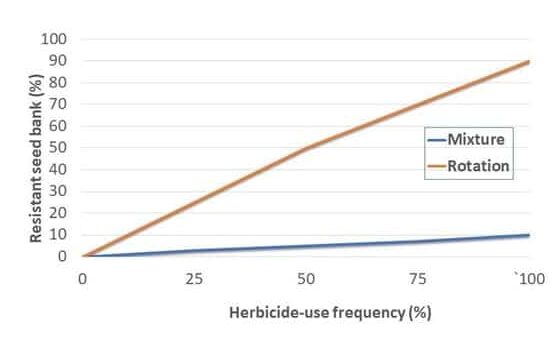
Crop rotation is a foundation of weed and other pest management practices. Since the management of one particular crop may allow weeds that need similar environments to survive, the rotation of various crops creates diverse environments over time, which helps suppress weed populations. Crop rotation also creates diverse environments through the use of different herbicide active ingredients. A 2-year rotation, such as corn-soybean, is a standard practice in many areas, but will not provide the same weed suppression benefits as a 3- or 4-year rotation.
Scouting and proper identification of waterhemp is important. Waterhemp looks similar to other pigweed species, but waterhemp is much more aggressive than most of these others. Since waterhemp is known to evolve herbicide resistance, a zero-tolerance approach is recommended irrespective of economic thresholds for management.
Cover crops can reduce weed populations. In order to increase cover crop effectiveness on a species like waterhemp with continuous germination, delay cover crop termination as late as possible. Cereal rye and mixtures with cereal rye are options to consider. In northern locations, cereal rye may need to be broadcasted into the standing crop in the fall before harvest in order to establish before frost. A typical seeding rate for cereal rye is 60 lbs/A, but may vary by locale and ranges from 30 to 110 lbs/A. Cereal rye should be terminated 10 to 14 days before planting corn, but for soybeans, cereal rye can be terminated after soybean planting (“planting green”) to allow maximum growth. Cereal rye may be terminated with herbicides and the crop may be planted into standing cereal rye, or cereal rye may be terminated by rolling the cover crop to create a mat of biomass into which the crop is planted.
Prevention is also an important but often overlooked management strategy. Clean mud-containing seeds from equipment and footwear before entering the field, harvest infested fields last, and buy certified seed when possible, to avoid weed seed contamination. Thoroughly clean the combine before harvest. Harvest herbicide-resistant weed-infested fields last. Plan your harvest ahead of time to avoid or minimize within the field and between field spread of weed seeds.

Mechanical weed control in the form of tillage or cultivation may be an effective method of control if done timely. Primary tillage can control seedlings. Strategic (or occasional) moldboard plowing can be used to bury seeds beyond the germination zone. Seeds need to emerge from near the soil surface; the hypocotyl can only elongate up to 1.4 inches. Moldboard plowing can place the seeds deeper than this and prevent seedling emergence. Cultivation is very effective, but only on small seedlings. Mature plants may re-root, making cultivation ineffective.
Cutting or mowing alone is not a good option for control and will result in branching from axillary buds on the stem or prostrate growth habit. Repeated mowing may reduce seed production but will not prevent it.
Harvest weed seed control (HWSC) tactics rely upon machinery modifications and the mechanical and/or cultural treatment of crop chaff at harvest to manage or destroy weed seeds. In order for HWSC to be effective, weed seeds must be retained on the plant at harvest, and waterhemp may retain more than 96% of seeds at soybean harvest, suggesting that this may be an effective practice for waterhemp control. HWSC has become an accepted practice for managing herbicide resistance in Australian cropping systems. There are six systems currently in use. One method, chaff lining, concentrates the crop chaff and weed seeds in a line behind the combine. Research is underway in the US to assess the effects of chaff lining on waterhemp emergence and seed decay. Impact mills, which shatter the seeds at high velocities, have been shown to be an effective tool to reduce viable seeds from entering the seed bank, and may destroy up to 99% of the weed seeds captured.
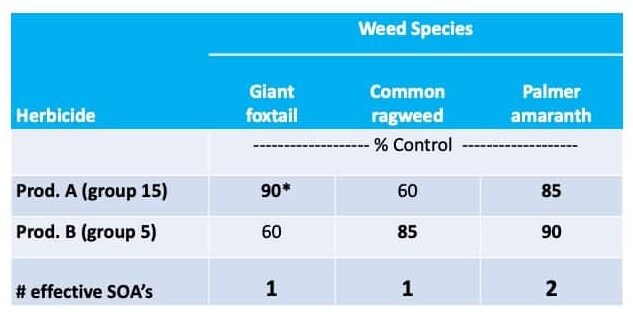
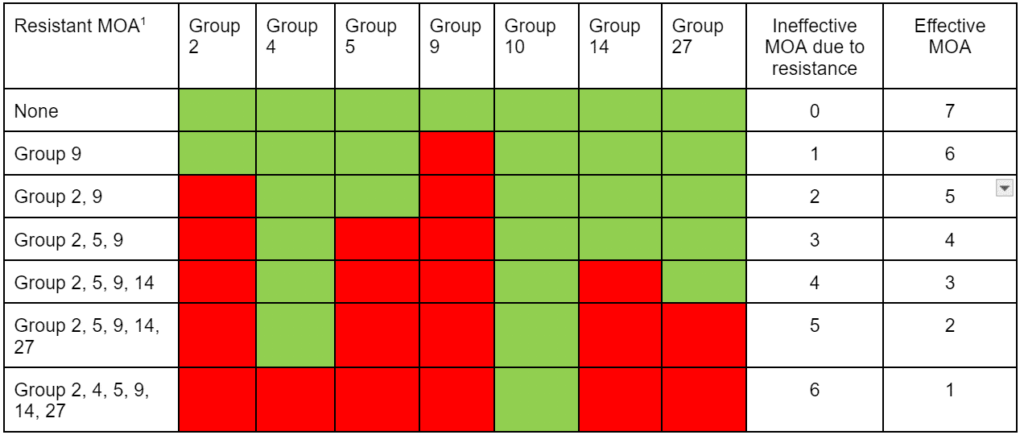
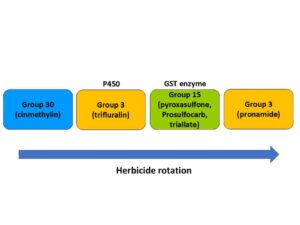
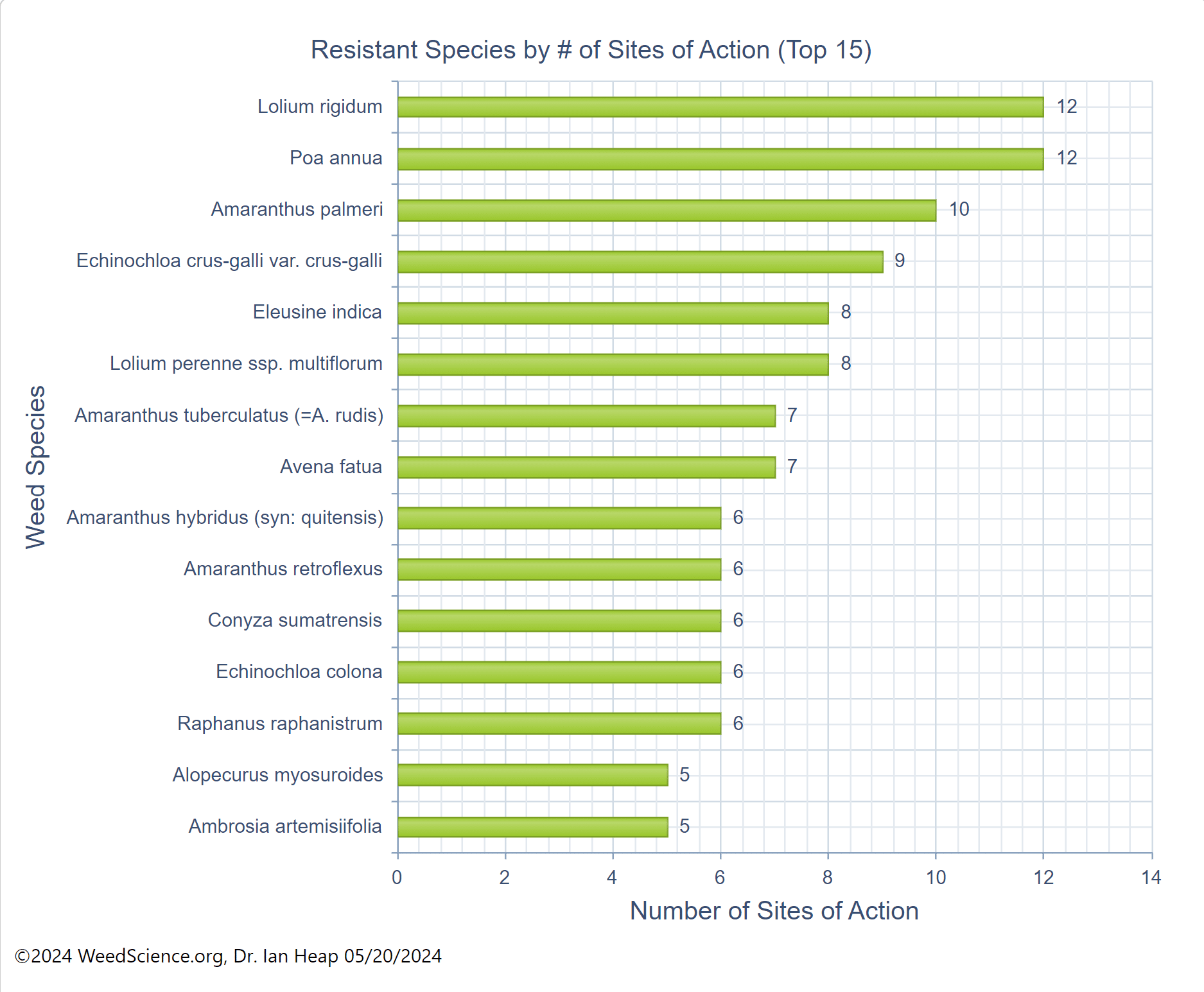
Chemical control of waterhemp has become problematic due to the frequency of herbicide resistance. It is important to know the resistance status of waterhemp populations in your region in order to design herbicide programs with multiple effective sites of action. Genes conferring herbicide resistance are known to spread with the movement of pollen, and therefore, resistance may spread quickly during reproductive periods. Multiple, effective sites of action should be used with each application, and sites of action should be rotated when possible. Applicators should always follow the herbicide label, use full-labeled rates, and apply in a timely manner.

Herbicides providing residual control are needed for waterhemp, since it has a long germination period. It is recommended to plant into a weed-free seedbed (with all existing weeds killed prior to planting) and use a preemergence, residual herbicide at planting, followed by a timely application of postemergence herbicides including ones that provide residual control. Preemergence or residual herbicides can protect crop yield by delaying waterhemp establishment early in the growing season.
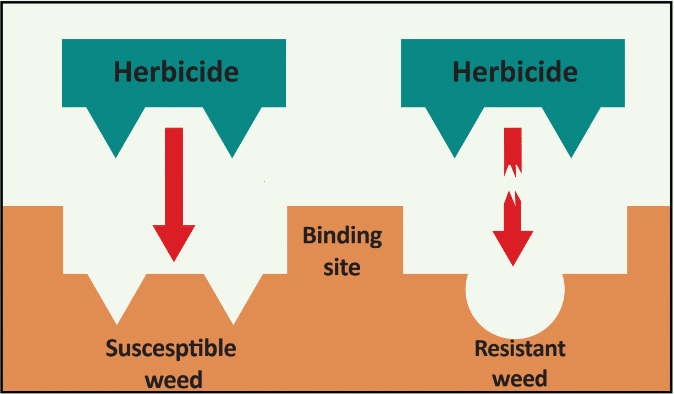
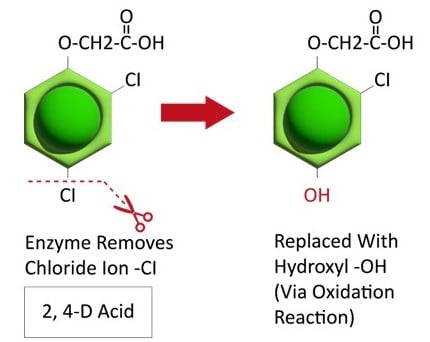
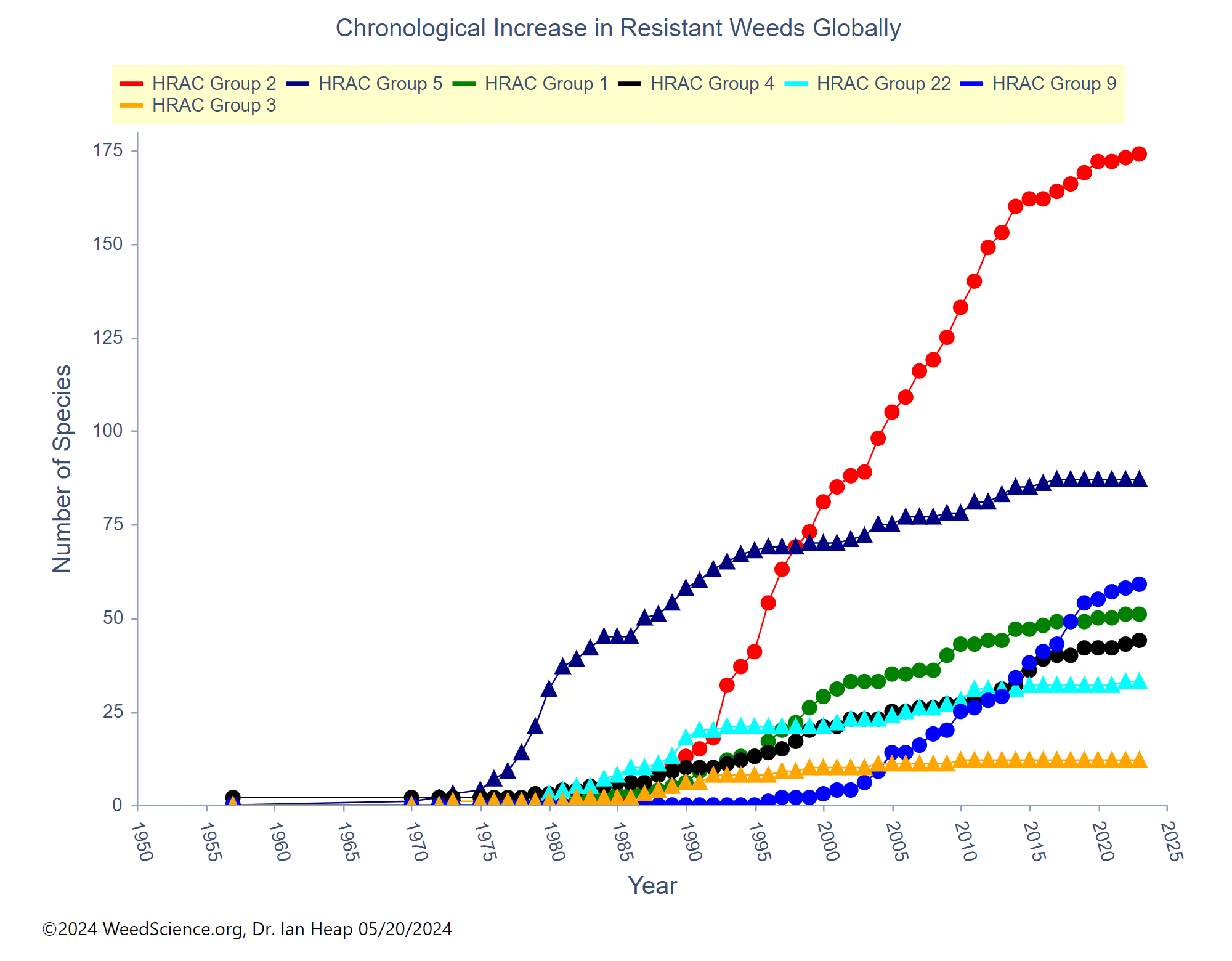
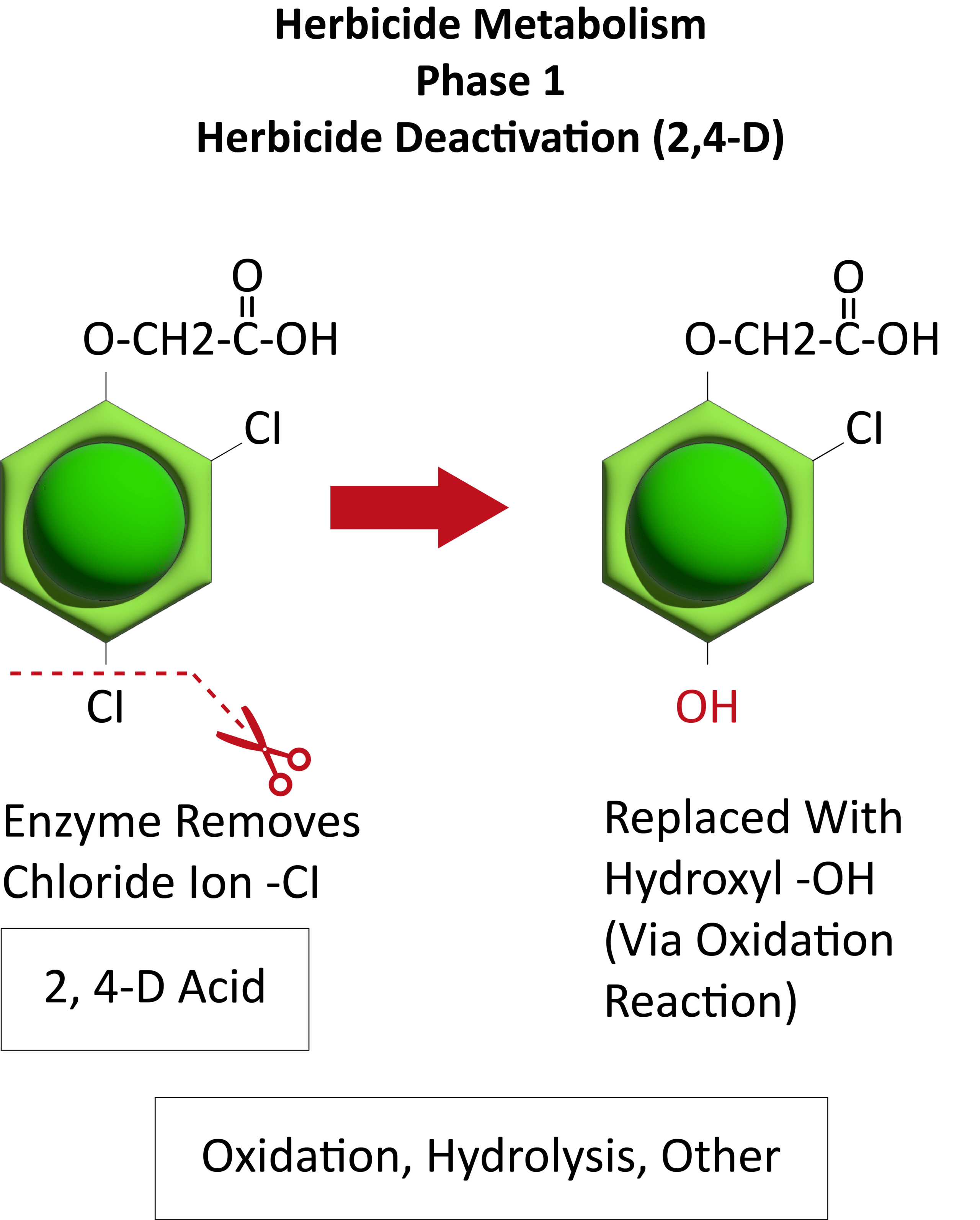
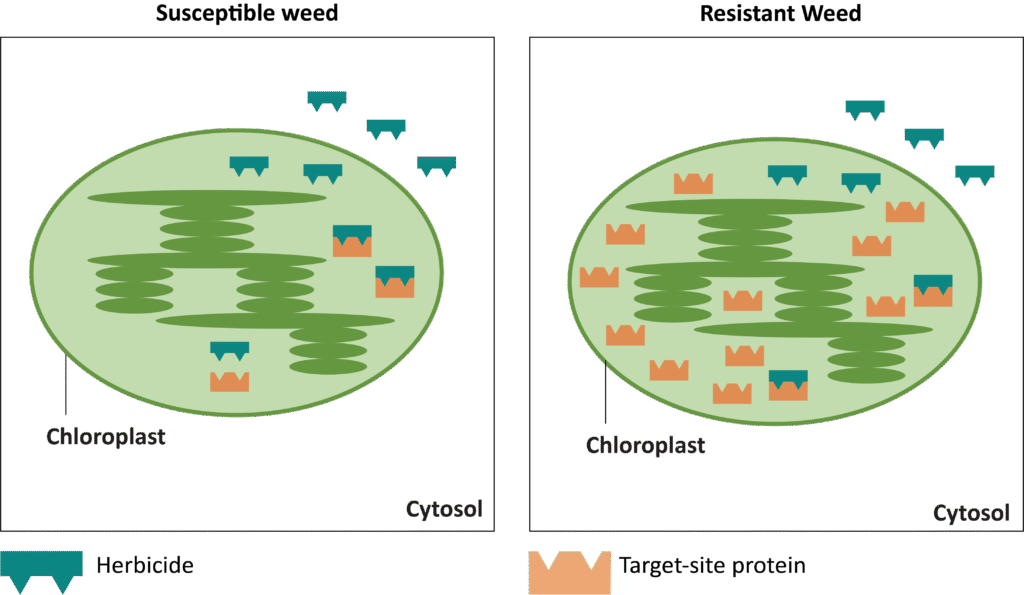
Waterhemp has evolved resistance to some common residual herbicides (see Graph 1). Depending upon the status of herbicide resistance in the field being treated, some examples of effective herbicides may include Group 3 [pendimethalin (Prowl H2O), trifluralin (Treflan)]; Group 5 [metribuzin (numerous), atrazine]; Group 7 [linuron (Lorox)]; Group 14 [fomesafen (Reflex), sulfentrazone (Authority products), flumioxazin (Valor)]; Group 15 [acetochlor (Harness/Warrant), pyroxasulfone (Zidua), and S-metolachlor (Dual II Magnum)]. Using products that are premixes of multiple effective ingredients or tank mixes from these groups is an important strategy for maximizing control and slowing the evolution of resistance. Target site resistance occurs when there is a genetic mutation that affects the herbicide binding site in the plant, causing the herbicide to lose effectiveness. Another type of resistance, non-target site resistance, is now being recognized. This type of resistance allows the plant to detoxify an herbicide and may confer resistance to a single herbicide active ingredient or multiple active ingredients across herbicide site of action groups. Effective strategies for managing non-target site resistance are being investigated.
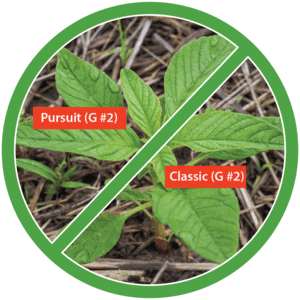
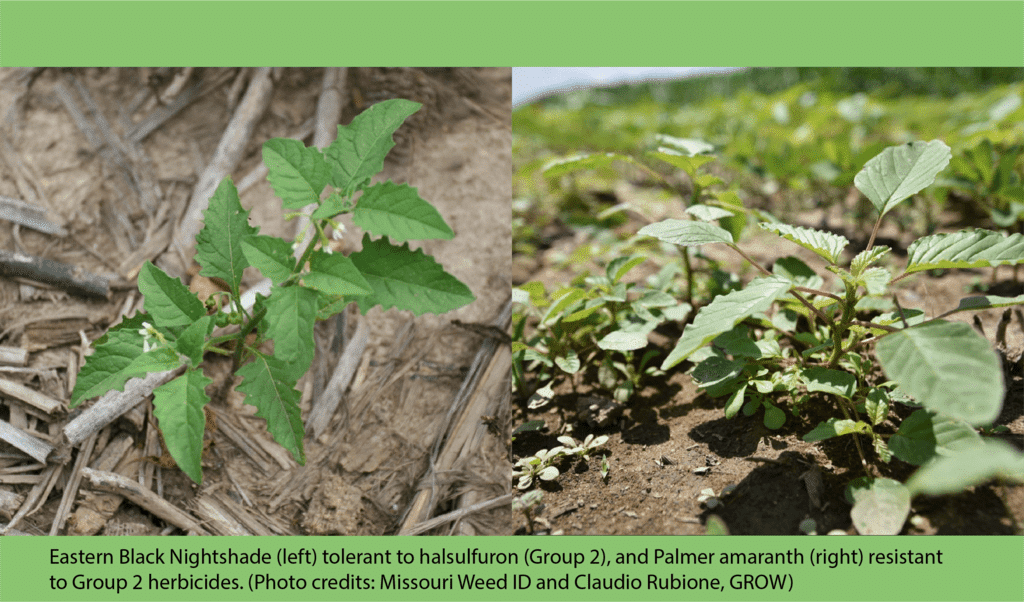
Postemergence control of waterhemp requires treatment with herbicide before it reaches four inches in height, and the plant may grow more than one inch per day. Resistance to Group 2 herbicides [imazamox (Raptor), chlorimuron (Classic)] is widespread and, therefore, should be assumed nationwide. Resistance to glyphosate (Roundup) and Group 14 herbicides [fomesafen (Reflex or Flexstar), lactofen (Cobra)] is also common. Group 14 herbicides can be used if resistance is not suspected and waterhemp is less than four inches tall; good spray coverage is critical. Group 27 herbicides [mesotrione (Callisto), tembotrione (Laudis), and topramazone (Impact or Armezon)] are highly effective when used in combination with atrazine. Postemergence control with Liberty Link crops, using glufosinate [Group 10 (Cheetah, Interline, Liberty)] is effective, if applications are made when waterhemp is less than 3-4 inches tall, with good coverage (15 GPA or more, medium droplets). In appropriate crops and with favorable environmental conditions, dicamba (i.e. Clarity, Diflexx, Engenia, XtendiMax), or 2,4-D (Enlist) can provide effective control. Postemergence herbicides should be applied with the addition of an overlapping soil residual herbicide, such as site of action Group 15 [acetochlor (Warrant), dimethenamid (Outlook), pyroxasulfone (Zidua), S-metolachlor (Dual II Magnum)].
Waterhemp plants that have survived a postemergence application and begin to regrow by two weeks after the application will need to be removed before they produce seed.
Refer to your local extension’s weed management guide for effective options. For more information on chemical waterhemp management, visit:
Biological control is difficult to implement. There are no approved biological controls for waterhemp. However, some ecological associations may help keep populations in check. The amaranth flea beetle (Disonycha glabrata) and others consume foliage, which can decrease seed production. Ground beetles (Carabidae) and crickets (Gryllidae) consume seeds. While release of these insects into a field is not economically feasible for management, judicious insecticide use is helpful to preserve these beneficials. These insect seed predators are more common in fields where there is residue or cover crops to use as shelter. The use of cover crops as habitat by seed predators may have a positive effect on the reduction of waterhemp populations with continued use of cover crops. Fungal pathogens may be potential biocontrol agents. For example, the fungus Microsphaeropsis amaranthi is effective in causing waterhemp death, but loses activity when tank mixed with herbicide.
Similar Weed Species
Waterhemp (Amaranthus tuberculatus) is closely related to other pigweed species, including Palmer amaranth (Amaranthus palmeri), redroot pigweed (Amaranthus retroflexus), smooth pigweed (Amaranthus hybridus), Powell pigweed (Amaranthus powellii), and spiny amaranth (Amaranthus spinosus). Palmer amaranth is more competitive, grows more quickly, and produces more seed than waterhemp, so proper identification is important. Other species may look similar but are of relatively less concern, since they are easier to control than waterhemp. The table below compares the characteristics of the most common pigweeds in North America.
| Characteristic | Palmer amaranth | Waterhemp | Spiny amaranth | Redroot pigweed* | Smooth pigweed* | Powell amaranth |
| Stem hairs | No | No | No | Yes | Yes | Yes |
| Leaf shape | diamond-shaped | long, narrow | egg-shaped | egg-shaped | egg-shaped | egg-shaped |
| Petiole length | long, often as long as leaf blade | medium, half the length of the leaf blade | medium, 1/2 inch spines where petioles attach to stem | short | short | short |
| Separate male/female plants | Yes | Yes | No | No | No | No |
| Seedhead | prickly, long, thick | smooth, long, slender | male flowers at top and female middle and bottom of the plant | prickly, short, stout | slightly prickly, long, slender | prickly, long, thick |
| Watermarks | Occasionally | No | Often | No | No | No |
*Separating redroot pigweed from smooth pigweed relies on sepal/tepal length at maturity:
-Sepals of redroot pigweed are about twice as long as the seed and often curved outward
-Sepals of smooth pigweed are the same length as seed
Authors
- Karla Gage
Editors
- Victoria Ackroyd
- Michael Flessner
- Lauren Lazaro
- Steven Mirsky
- Claudio Rubione
- Mark VanGessel
Reviewed by
- Aaron Hager
- Amit Jhala
Resources
Heap I (2021) The International Herbicide-Resistant Weed Database www.weedscience.org Accessed: April 6, 2021.
Take action on Weeds: Waterhemp https://growiwm.org/factsheets/waterhemp-management-fact-sheet/. Accessed: May 18, 2021
USDA Plants Database. Amaranthus tuberculatus (Moq.) Sauer https://plants.usda.gov/core/profile?symbol=AMTU Accessed: May 29, 2020
Citations
Bensch CN, Horak MJ, Peterson D (2003) Interference of redroot pigweed (Amaranthus retroflexus), Palmer amaranth (A. palmeri), and common waterhemp (A. rudis) in soybean. Weed Science 51:37–43 doi.org/10.1614/0043-1745(2003)051[0037:IORPAR]2.0.CO;2
Burnside OC, Wilson RG, Weisberg S, Hubbard KG (1996) Seed longevity of 41 weed species buried 17 years in eastern and western Nebraska. Weed Science 44:74–86 www.jstor.org/stable/4045786
Costea M, Weaver SE and Tardif FJ (2004) Biology of Canadian weeds 130. Amaranthus retroflexus L., A. powellii S. Watson and A. hybridus L. Canadian Journal of Plant Science 84:631–668 doi.org/10.4141/P02-183
Costea, M, Weaver, SE, Tardif, FJ (2005) The biology of invasive alien plants in Canada. 3. Amaranthus tuberculatus (Moq.) Sauer var. rudis (Sauer) Costea & Tardif. Canadian Journal of Plant Science 85:507-522 doi.org/10.4141/P04-101
Davis AS, Hill JD, Chase CA, Johanns AM, Liebman M (2012) Increasing cropping system diversity balances productivity, profitability and environmental health. PLoS ONE 7:e47149 doi.org/10.1371/journal.pone.0047149
Farmer JA, Webb EB, Pierce RA, Bradley KW (2017) Evaluating the potential for weed seed dispersal based on waterfowl consumption and seed viability. Pest Management Science 73:2592-2603 doi.org/10.1002/ps.4710
Fisk JW, Hesterman OB, Shrestha A, Kells JJ, Harwood RR, Squire JM, Sheaffer CC (2001) Weed suppression by annual legume cover crops in no-till corn. Agronomy Journal 93:319–325 doi.org/10.2134/agronj2001.932319x
Franca LX (2015) Emergence patterns of common waterhemp and Palmer amaranth in southern Illinois. Theses. Paper 1756 https://opensiuc.lib.siu.edu/theses/1756/
Green JK, Norsworthy JK, Walsh MJ (2020) Distribution of common cocklebur and Palmer amaranth seed exiting the combine for harvest weed seed control in soybean. Crop, Forage & Turfgrass Management 6:p.e20064 doi.org/10.1002/cft2.20064
Guo J, Riggins CW, Hausman NE, Hager AG, Riechers DE, Davis AS, Tranel PJ (2015) Nontarget-site resistance to ALS inhibitors in waterhemp (Amaranthus tuberculatus). Weed Science 63:399-407 doi.org/10.1614/WS-D-14-00139.1
Hager AG, Wax LM, Stoller EW, Bollero GA (2002) Common waterhemp (Amaranthus rudis) interference in soybean. Weed Science 50:607–610 doi.org/10.1614/0043-1745(2002)050[0607:CWARII]2.0.CO;2
Jones EAL, Owen MDK (2021) Investigating the efficacy of selected very-long-chain fatty acid-inhibiting herbicides on Iowa waterhemp (Amaranthus tuberculatus) populations with evolved multiple herbicide resistances. Agronomy 11:595 doi.org/10.3390/agronomy11030595
Jugulam M, Shyam C (2019) Non-target-site resistance to herbicides: recent developments. Plants 8:417 doi.org/10.3390/plants8100417
Korres NE, Norsworthy JK, Young BG, Reynolds DB, Johnson WG, Conley SP, Smeda RJ, Mueller TC, Spaunhorst DJ, Gage KL, Loux M (2018) Seedbank persistence of Palmer amaranth (Amaranthus palmeri) and waterhemp (Amaranthus tuberculatus) across diverse geographical regions in the United States. Weed Science 66:446-56 doi.org/10.1017/wsc.2018.27
Refsell DE, Hartzler RG (2009) Effect of tillage on common waterhemp (Amaranthus rudis) emergence and vertical distribution of seed in the soil. Weed Technology 23:129-133 doi.org/10.1614/WT-08-045.1 doi.org/10.1038/srep44913
Sauer JD (1955) Revision of the dioecious amaranths. Madroño 13:5–46 www.jstor.org/stable/41422838
Schwartz-Lazaro LM, Green JK, Norsworthy JK (2017) Seed retention of Palmer amaranth (Amaranthus palmeri) and barnyardgrass (Echinochloa crus-galli) in soybean. Weed Technology 31:617-622 doi.org/10.1017/wet.2017.25
Schwartz-Lazaro LM, Norsworthy JK, Walsh MJ, Bagavathiannan MV (2017) Efficacy of integrated Harrington seed destructor on weeds of soybean and rice production systems in the southern United States. Crop Science 57:2812-2818 doi.org/10.2135/cropsci2017.03.0210
Smith DA, Hallett SG (2004) Interactions between Microsphaeropsis amaranth and glyphosate for the control of waterhemp, Amaranthus tuberculatus. Weed Science Society of America Annual Meeting. 2004 Feb. 07–11. Kansas City, MO. Vol. 44, p. 73 (Abstr.)
Steckel LE, Sprague CL (2004) Common waterhemp (Amaranthus rudis) interference in corn. Weed Science 52:359–364 doi.org/10.1614/WS-03-066R1
Ulloa SM, Datta A, Knezevic SZ (2010) Growth stage-influenced differential response of foxtail and pigweed species to broadcast flaming. Weed Technology 24:319-325 doi.org/10.1614/WT-D-10-00005.1
Waselkov KE, Regenold ND, Lum RC, Olsen KM (2020) Agricultural adaptation in the native North American weed waterhemp, Amaranthus tuberculatus (Amaranthaceae). PloS one 15: p.e0238861 doi.org/10.1371/journal.pone.0238861