Ambrosia trifida L.
Also known as great ragweed

Biology
Due to giant ragweed’s early emergence, rapid growth and biomass accumulation, high photosynthetic rate, and ability to adapt to diverse environments, it can quickly outgrow and outcompete the crop and other weeds for resources such as light. Giant ragweed plants have deeply lobed leaves which typically consist of 3 lobes; however, plants have been observed to display both fewer and more lobes depending on the growing environment. Giant ragweed is capable of rapid growth and can reach up 17 feet in height. Giant ragweed’s ability to accumulate biomass and reach considerable heights allow this plant to compete aggressively with crops. Research has shown that 2 giant ragweed plants in 110 ft2 can reduce corn yield by 13% while only 1 plant in that same area can reduce soybean yield by 50%.
Where is giant ragweed a problem?
Giant ragweed is found throughout the eastern two-thirds of the US in agricultural fields, stream banks, and edge habitats such as grassways and fencerows. Historically, giant ragweed was primarily a weed found along stream banks and was not a major problem in row crop production. Widespread reliance on tillage has resulted in giant ragweed becoming a common, troublesome weed in row crop fields, particularly corn and soybean.
If control measures are not performed shortly after giant ragweed emerges, it can quickly outgrow and shade the crop. Giant ragweed is capable of producing late-emerging, shade tolerant axillary leaves which further increase competition for light and soil moisture.
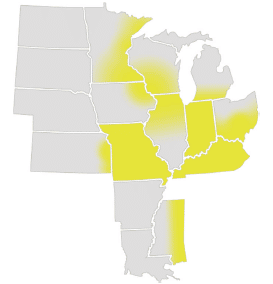
Estimated distribution of giant ragweed (yellow) across soybean production areas based on the perspectives of regional weed science specialists. Graphic from the “Weed and Weed Seed Challenges in U.S. Soybeans” report, used with permission from the United Soybean Board.
What is the emergence pattern of giant ragweed?
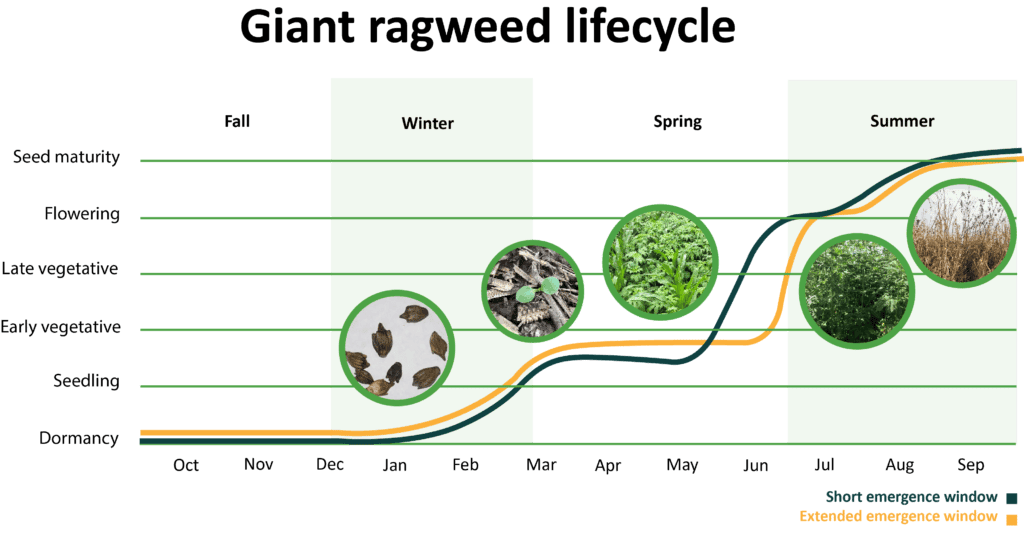
There are two giant ragweed biotypes with distinct emergence patterns. Giant ragweed populations found in western Iowa, Minnesota, and Nebraska tend to emerge from March to June with 90% emergence predicted by mid-May (short emergence window). On the other hand, populations found in Illinois, Indiana, Ohio, and Wisconsin continue to emerge later into the growing season (extended emergence window). In fields where the biotype with the extended emergence window is present, giant ragweed is particularly difficult to control and can require several sequential herbicide applications. Giant ragweed’s large seed size allows it to emerge from deep in the soil and emergence is often stimulated by tillage.

What is the life cycle of giant ragweed like?
Giant ragweed is one of the earliest emerging summer annual weeds. The first seedlings often emerge in March to April. After emergence, giant ragweed grows rapidly.
Giant ragweed is monoecious (both male and female flower parts on the same plant). However, plants are more prone to cross pollination than self-pollination, allowing genetic traits such as herbicide resistance to quickly spread in a population. Plants bloom from July to October producing excessive amounts of pollen and seeds that remain on the plants until maturity and plant death.
Giant ragweed requires a period of cold to break seed dormancy and allow it to germinate.
How does giant ragweed spread?
As a summer annual giant ragweed plants disperse solely through seed production. The relatively large size of giant ragweed seeds limits its ability to be dispersed by wind. Seeds can be moved within field and from field to field through tillage and harvest equipment. Additionally, giant ragweed is heavily predated by rodents and birds post-dispersal which can inevitably lead to movement of seed when the seed is not destroyed.
How many seeds can giant ragweed produce and how long can those seeds survive?
Giant ragweed can produce 500 to 5,000 seeds per plant; however, typically only 60 – 70 % are viable at plant maturity. Research conducted in Minnesota observed that roughly 80% of seeds produced remained on giant ragweed plants into October, demonstrating that the majority of seed is retained through the typical soybean harvest period. As the majority of giant ragweed seeds remain on plants through harvest, combines can serve as dispersal mechanisms. Additionally, giant ragweed seed contamination of grain is common in US corn and soybean production due to its large seed size. A recent survey conducted by the United Soybean Board indicated giant ragweed seeds were present in 25% of the soybean samples containing weed seeds.

What other biological weaknesses does giant ragweed have that can be targeted with management techniques?
Adopting no-till increases the likelihood of seeds being predated or decaying on the soil surface. Furthermore, effective control integrating mechanical, cultural, and chemical approaches up front will draw down the seedbank and lead to a population density that is much more manageable over time. In general, short emergence window giant ragweed biotypes will be easier to effectively control than extended emergence window biotypes. A large portion of short emergence window biotypes can be controlled with an effective chemical herbicide burndown application or timely pre-plant tillage. In this scenario, the few giant ragweed plants that emerge after these tactics can then be controlled with a timely, effective post-emergence herbicide application.
Herbicide Resistance
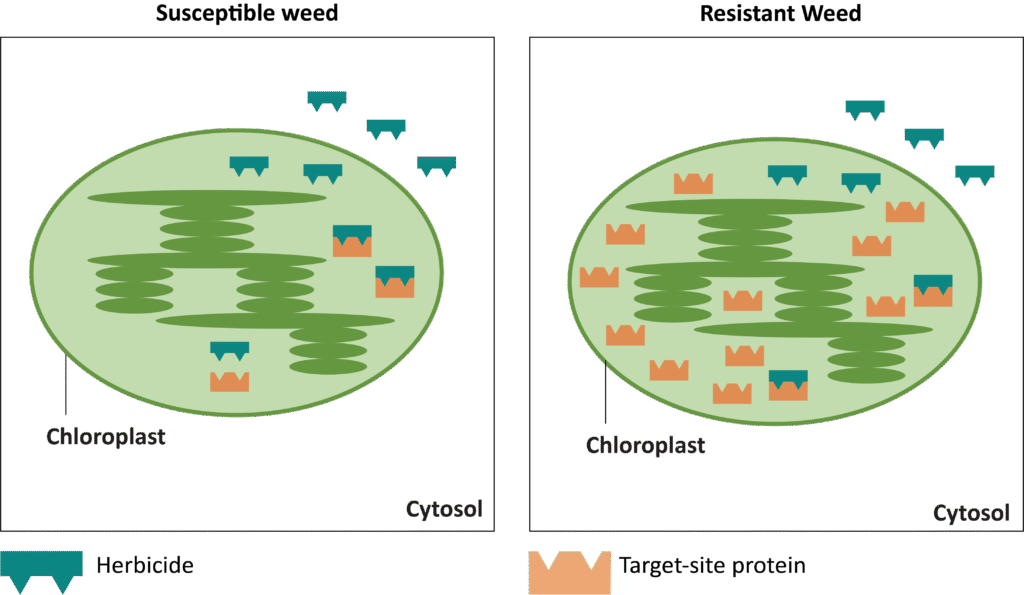
Glyphosate-resistant giant ragweed has been documented to have two different resistance mechanisms: rapid-response (RR) and non-rapid response (NRR). The RR biotype responds to glyphosate with rapid necrosis in mature leaves while immature leaves turn chlorotic within 2-6 hours. Plants then subsequently resume normal growth within a few days. Conversely, leaves of the NRR biotype become chlorotic at the apical meristem and growth is stunted. Normal growth resumes within 1-2 weeks after glyphosate application.
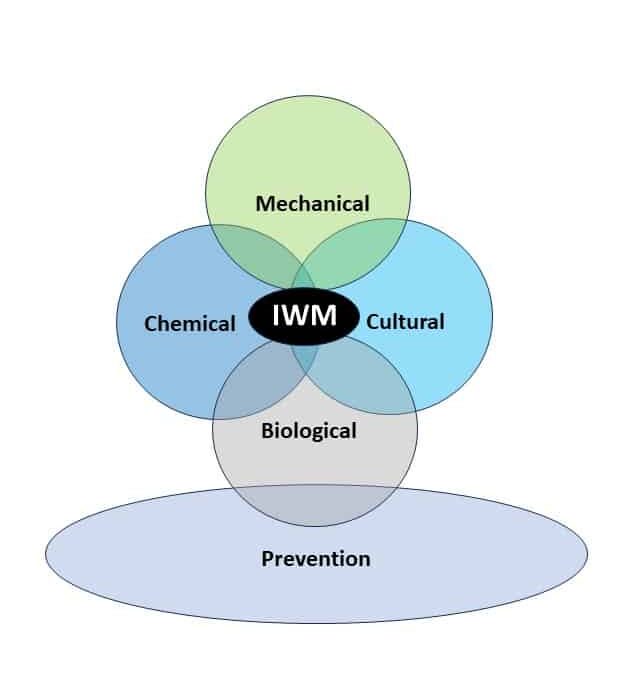
Integrated Weed Management Strategies
Cultural Tactics: There are several cultural practices that can be employed to help control giant ragweed. Delaying crop planting date to allow more of the giant ragweed to emerge before pre-plant tillage or a burndown herbicide application has improved overall control. Also, later planting can improve the crop’s ability to compete. Since giant ragweed can continue to emerge after cash crops are planted, narrow row spacing can increase soybean competitive ability and initially slow weed growth. Rotating crops allows for different levels of competitiveness each year as crops often emerge at different times. Increased crop competitiveness alone is not an adequate method for giant ragweed control; however, it can help extend the available window for effective postemergence applications. Crop rotation also permits use of a greater diversity of herbicides to control giant ragweed, which reduces the selection pressure for herbicide resistance. Adoption of no-till or reduced tillage practices can also favor giant ragweed seed decay and predation.
As was indicated previously, giant ragweed retains the majority of its seed through harvest. Routine cleaning of a combine between fields can help decrease the spread of giant ragweed from infested fields to uninfested fields. Additionally, following a harvest sequence that begins with uninfested fields and ends with fields with high infestations or known herbicide-resistant populations can also further slow the spread of giant ragweed. This approach should be used with tillage equipment and tillage sequence, as well.
Cover Crops: There is limited research indicating effective control of large seeded weeds such as giant ragweed with cover crops.
Mechanical: Tillage should be performed in the spring, prior to planting, but not in the fall. Fall tillage can bury giant ragweed seeds, increasing their chance of survival by reducing seed decay and predation. However, spring tillage can serve as a tool for managing giant ragweed that emerges before crop planting. Research conducted in Nebraska indicated pre-plant tillage provided > 90% control of giant ragweed seedlings 14 days after treatment; however, additional post-emergence herbicide applications were needed for season-long control. Timing of spring tillage is particularly important as it does not alter giant ragweed emergence windows and, if performed too early (ahead of the bulk of giant ragweed emergence), will result in ineffective control.
The high seed retention of giant ragweed makes it a good candidate for harvest weed seed control (HWSC). Research conducted in Illinois observed nearly 97% of giant ragweed seeds were killed when processed through a seed impact mill at harvest. When post-harvest resources are not available, pre-harvest hand roguing of giant ragweed plants could serve as a viable management strategy to decrease or prevent replenishment of the seedbank.
Giant ragweed commonly proliferates on the edges of agricultural fields. Management strategies for control of giant ragweed can be effective within the crop field, but if plants along the field edges are allowed to produce seeds, reinfestation of the field is common. Additional tactics such as mowing, hand roguing, and spot spraying giant ragweed along field margins can help prevent seed production and spread.
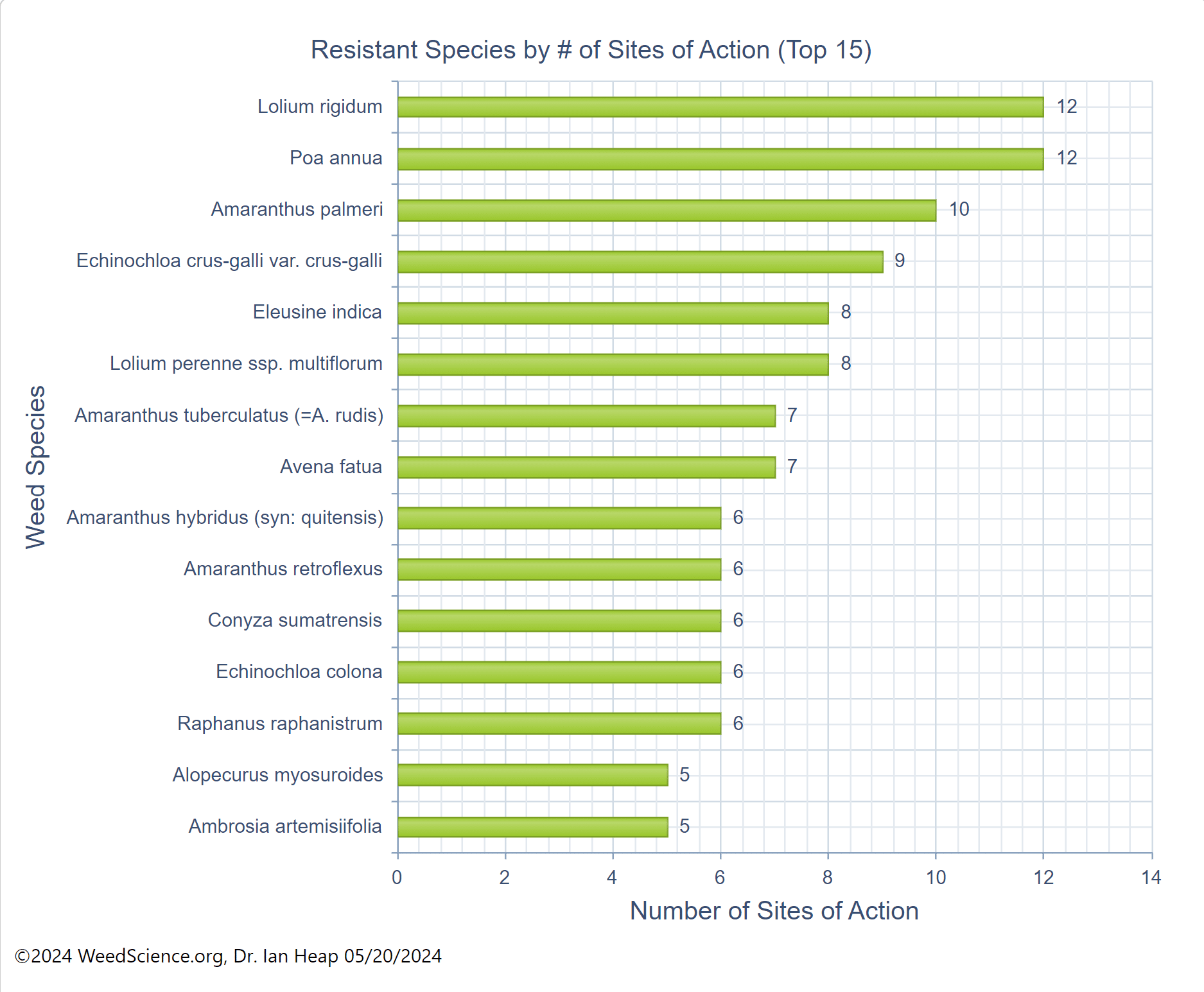
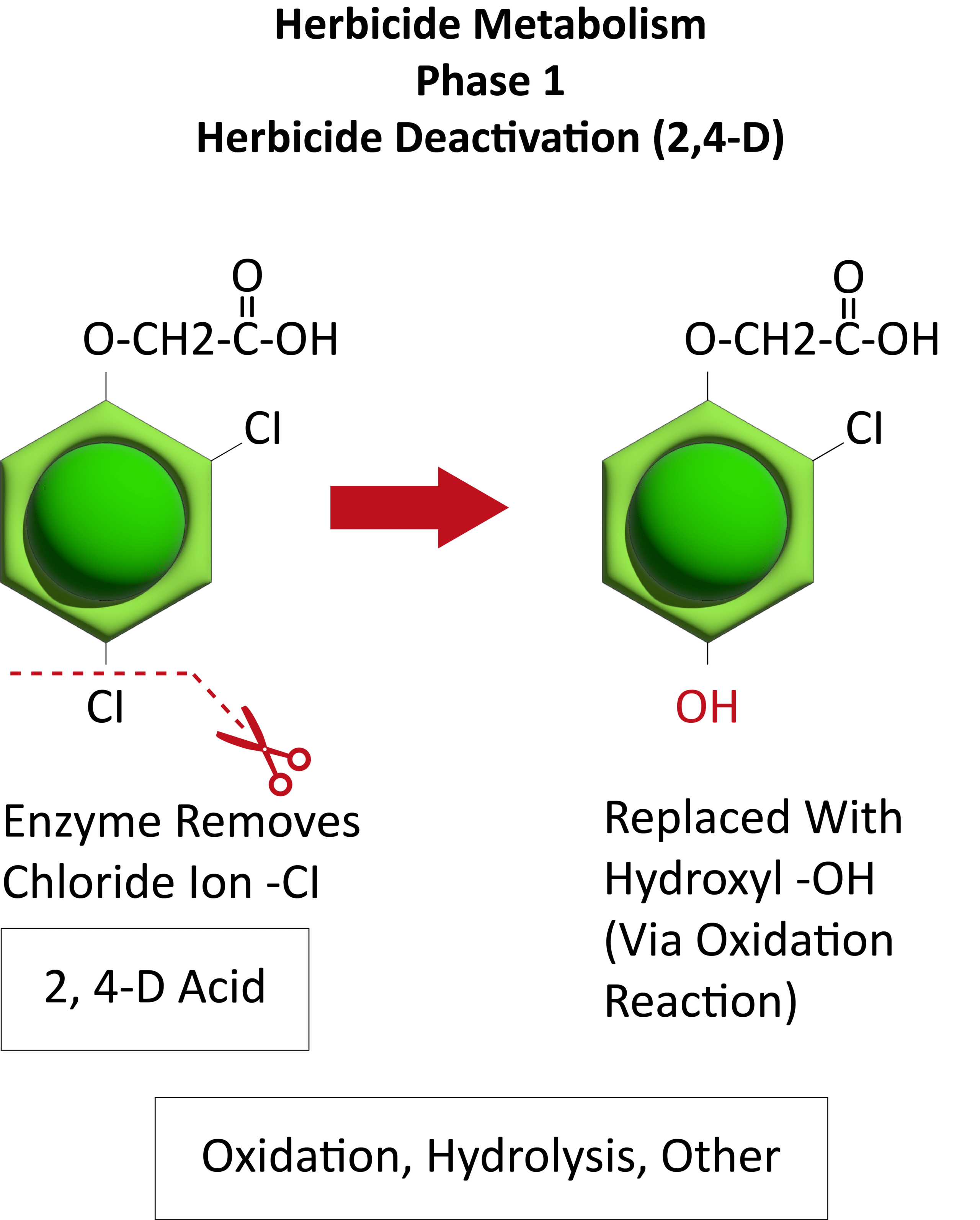
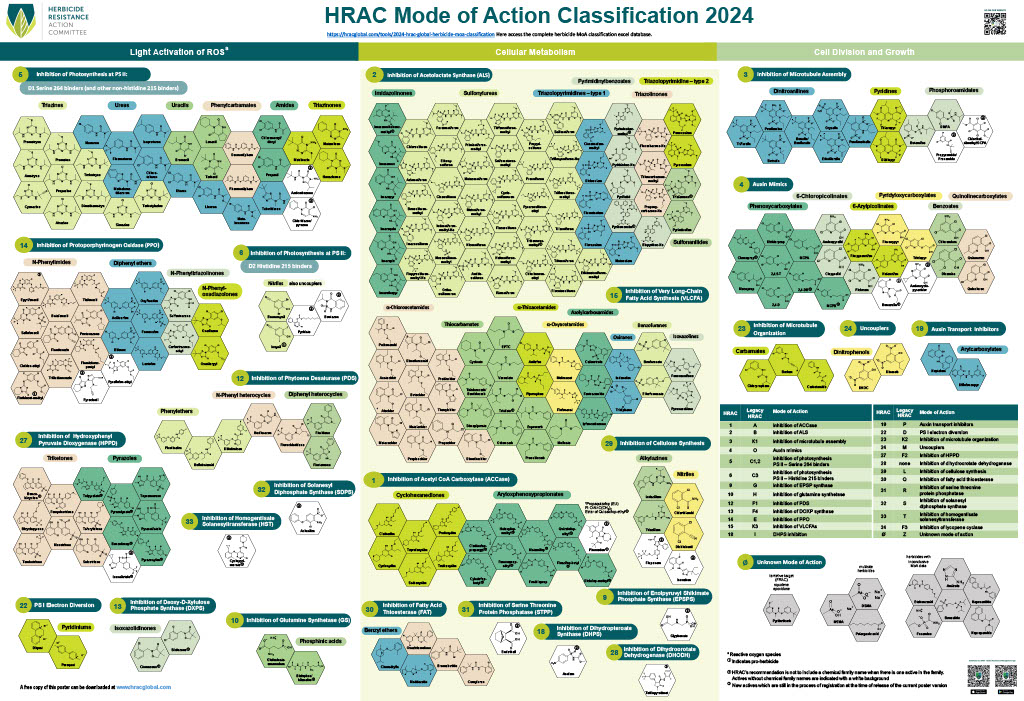
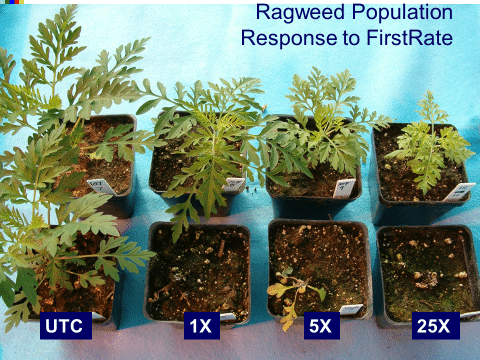
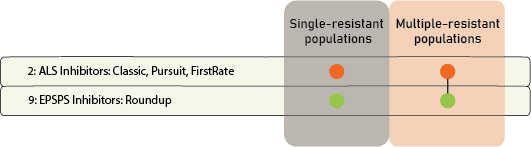
Chemical: Historically, ALS-inhibitors (Group 2) have been the foundation for giant ragweed control through burndown of established plants and subsequent soil-residual control. Glyphosate (Group 9) has served as another effective option for giant ragweed control in glyphosate-resistant crops. However, Group 2- and Group 9- resistant giant ragweed populations have been documented across the Midwest US, including populations with resistance to both groups documented in Ohio, Minnesota, Missouri, and Indiana.
For effective chemical control of giant ragweed it is important to start weed-free at planting. Using 2,4-D or dicamba (Group 4) combined with glyphosate provides an effective burndown of giant ragweed. Additionally, preemergence (PRE) herbicides with residual activity containing ALS-inhibitors (chlorimuron or cloransulam) and PPO-inhibitors (Group 14; fomesafen, flumioxazin, or sulfentrazone) can provide additional control of later emerging ALS-sensitive giant ragweed plants. PPO-inhibitors can provide inconsistent residual control of ALS-resistant giant ragweed populations. If relying on products containing fomesafen for PRE control of giant ragweed, be sure to pay attention to the restrictions of use rates for post-emergence applications. Due to its large seed size, overall efficacy of PRE herbicides is reduced for giant ragweed compared to small-seeded broadleaf weed species.
Dense populations of giant ragweed will most often require multiple post-emergence applications. The first POST application should be based on maximum weed size according to the product’s label (ie. generally 4-6 leaf stage or 3-6 inches tall) with the second POST timing 3-4 weeks later. POST control options for giant ragweed are dependent on the current crop and its respective herbicide-resistance traits, as well as the presence/absence of resistant weed populations.
As stated above, if giant ragweed populations are not resistant, ALS-inhibitors and glyphosate remain an effective POST option. PPO-inhibitors (fomesafen and lactofen) can be used for POST control of giant ragweed in soybeans regardless of herbicide-resistance trait. Glufosinate (Group 10) is an additional post-emergence option for glufosinate-resistant crops.
There are several options available for use in LibertyLink corn/soybeans and LLGT27 or Enlist E3TM soybeans. Enlist E3TM soybeans allow for 2,4-D-choline to be used POST while Xtend soybean allows for dicamba POST for giant ragweed control. In traited crops, 2,4-D and dicamba as POST create the opportunity for additional diversity in herbicide sites of action.
Since effective chemical management of giant ragweed requires multiple applications within a season, it is important to be mindful of the sites of action being used and the maximum amounts of individual active ingredients allowed within a given season.
Biological: Rodents, invertebrates, and insect larvae play an important role as biological control agents of giant ragweed. Research conducted in Ohio indicated that insect larvae accounted for up to 19% of seed viability losses before seed dispersal. Additional research from Ohio indicated that 88% of giant ragweed seeds were lost over the course of one calendar year through predation by rodents and invertebrates. Giant ragweed plants in no-till fields are more susceptible to seed predation as seeds remain on the soil surface for extended periods of time.
Limited research has been conducted evaluating a fungal pathogen of giant ragweed for biological control uses; however, low levels of infectivity have discouraged any further research on this treatment.
Similar Weed Species
Common ragweed (Ambrosia artemisiifolia L.) is of the same genus, Ambrosia, as giant ragweed and is also found in agricultural fields and edge habitats. Both ragweed species are somewhat similar in appearance; however, common ragweed has deeply lobed leaves compared to giant ragweed’s shallowly lobed leaves.


Authors
- Nick Arneson, University of Wisconsin-Madison
- Sarah Striegel, University of Wisconsin- Madison
- Rodrigo Werle, University of Wisconsin-Madison
Editors
- Victoria Ackroyd
- Mark VanGessel
- Michael Flessner
- Claudio Rubione
Reviewed by
- Mark Loux, Ohio State University
- J.D. Green, University of Kentucky
Resources
Take Action: Common Ragweed: https://iwilltakeaction.com/weed/giant-ragweed Accessed: July 15, 2021
Citations
Cartwright RD, Templeton GE (1988) Biological limitations of Protomyces gravidus as a mycoherbicide for giant ragweed, Ambrosia trifida. Plant Disease 72:580-582. doi.org/10.1094/PD-72-0580
Davis AS, Cardina J, Forcella F, Johnson GA, Kegode G, Lindquist JL, Luschei EC, Renner KA, Sprague CL, Williams II MM (2005) Environmental factors affecting seed persistence of annual weeds across the U.S. corn belt. Weed Sci 53:860-868. doi.org/10.1614/WS-05-064R1.1
Davis AS, Clay S, Cardina J, Dille A, Forcella F, Lindquist J, Sprague C (2013) Seed burial physical environment explains departures from regional hydrothermal model of giant ragweed (Ambrosia trifida) seedling emergence in U.S. Midwest. Weed Sci 61:415-421. doi.org/10.1614/WS-D-12-00139.1
Dickerson CT, Sweet RD (1971) Common ragweed ecotypes. Weed Sci 19:64-66 doi.org/10.1017/S0043174500048281
Ganie ZA, Kaur S, Jha P, Kumar V, Jhala AJ (2018) Effect of late season herbicide applications on inflorescence and seed production of glyphosate-resistant giant ragweed (Ambrosia trifida). Weed Technol 32:159-165. doi.org/10.1017/wet.2017.101
Ganie ZA, Sandell LD, Jugulam M, Kruger GR, Marx DB, Jhala AJ (2016) Integrated management of glyphosate-resistant giant ragweed (Ambrosia trifida) with tillage and herbicides in soybean. Weed Technol 30:45-56. doi.org/10.1614/WT-D-15-00089.1
Goplen JJ, Sheaffer CC, Becker RL, Coulter JA, Breitenbach FR, Behnken LM, Johnson GA, Gunsolus JL (2016) Giant ragweed (Ambrosia trifida) seed production and retention in soybean and field margins. Weed Technol 30:246-253. doi.org/10.1614/WT-D-15-00116.1
Goplen JJ, Sheaffer, CC, Becker RL, Coulter JA, Breitenbach FR, Behnken LM, Johnson GA, Gunsolus JL (2017) Seedbank depletion and emergence patterns of giant ragweed (Ambrosia trifida) in Minnesota cropping systems. Weed Sci 65:52-60. doi.org/10.1614/WS-D-16-00084.1
Goplen JJ, Sheaffer CC, Becker RL, Moon RD, Coulter JA, Breitenbach FR, Behnken LM, Gunsolus JL (2018) Giant ragweed (Ambrosia trifida) emergence model performance evaluated in diverse cropping systems. Weed Science 66:36-46. doi.org/10.1017/wsc.2017.38
Harre NT, Nie H, Robertson RR, Johnson WG, Weller SC, Young BG (2017) Distribution of herbicide-resistant giant ragweed (Ambrosia trifida) in Indiana and characterization of distinct glyphosate-resistant biotypes. Weed Sci 65:699-709. doi.org/10.1017/wsc.2017.56
Harrison SK, Regnier EE, Schmoll JT, Webb JE (2001) Competition and fecundity of giant ragweed in corn. Weed Sci 49:224-229. doi.org/10.1614/0043-1745(2001)049[0224:CAFOGR]2.0.CO;2
Harrison SK, Regnier EE, Schmoll JT (2003) Post Dispersal predation of giant ragweed (Ambrosia trifida) seed in no-tillage corn. Weed Sci 51:955-964. doi.org/10.1614/P2002-110
Harrison SK, Regnier EE, Schmoll JT, Harrison JM (2007) Seed size and burial effects on giant ragweed (Ambrosia trifida) emergence and seed demise. Weed Sci 55:16-22. doi.org/10.1614/WS-06-109.1
Liebman M, Nicholas VA (2020) Cropping system redesign for improved weed management: a modeling approach illustrated with giant ragweed (Ambrosia trifida) Agronomy 10:262. doi.org/10.3390/agronomy10020262
Moretti ML, Van Horn CR, Robertson RR, Segobye K, Weller SC, Young BG, Johnson WG, Sammons RD, Wang D, Ge X, d’Avignon A, Gaines TA, Westra P, Green AC, Jeffery T, Lesperance MA, Tardif FJ, Sikkema PH, Hall C, McLean MD, Lawton MB, Schulz B (2018) Glyphosate resistance in Ambrosia trifida: II. Rapid response physiology and non-target site resistance. Pest Manag Sci 74:1079-1088. doi.org/10.1002/ps.4569
Norsworthy JK, Jha P, Steckel LE, Scott RC (2010) Confirmation and control of glyphosate-resistant giant ragweed (Ambrosia trifida) in Tennessee. Weed Technol 24:64-70. doi.org/10.1614/WT-D-09-00019.1
Norsworthy JK, Riar D, Jha P, Scott RC (2011) Confirmation, control, and physiology of glyphosate-resistant giant ragweed (Ambrosia trifida) in Arkansas. Weed Technol 25:430-435. doi.org/10.1614/WT-D-10-00155.1
Regnier EE, Harrison SK, Loux MM, Holloman C, Venkatesh R, Diekmann F, Taylor R, Ford RA, Stoltenberg DE, Hartzler RG, Davis AS, Schutte BJ, Cardina J, Mahoney KJ, Johnson WG (2016) Certified Crop Advisors’ perceptions of giant ragweed (Ambrosia trifida) distribution, herbicide resistance, and management in the corn belt. Weed Sci 64:361-377. doi.org/10.1614/WS-D-15-00116.1
Schutte BJ, Regnier EE, Harrison SK, Schmoll JT, Spoka K, Forcella F (2008) A Hydrothermal seedling emergence model for giant ragweed (Ambrosia trifida) Weed Sci 56:555-560. doi.org/10.1614/WS-07-161.1
Schutte BJ, Regnier EE, Harrison SK (2012) Seed dormancy and adaptive seedling emergence timing in giant ragweed (Ambrosia trifida). Weed Sci 60:19-26. doi.org/10.1614/WS-D-11-00049.1
Shergill LS, Bejleri K, Davis A, Mirsky SB (2020) Fate of weed seeds after impact mill processing in midwestern and mid Atlantic United States. Weed Sci 68:92-97. doi.org/10.1017/wsc.2019.66
Van Horn CR, Moretti ML, Robertson RR, Segobye K, Weller SC, Young BG, Johnson WG, Schulz B, Green AC, Jeffery T, Lesperance MA, Tardif FJ, Sikkema PH, Hall C, McLean MD, Lawton MB, Sammons RD, Wang D, Westra P, Gaines TA (2017) Glyphosate resistance in Ambrosia trifida: I. Novel rapid cell death response to glyphosate. Pest Manag Sci 74:1071-1078. doi.org/10.1002/ps.4567
Webster TM, Loux MM, Regnier EE, Harrison SK (1994) Giant ragweed (Ambrosia trifida) canopy architecture and interference studies in soybean (Glycine max). Weed Technol 8:559-564. doi.org/10.1017/S0890037X00039683
Werle R, Sandell LD, Buhler DD, Robert G, Lindquist JL, Werle R, Sandell LD, Buhler DD, Hartzler RG, Lindquist JL (2014) Predicting emergence of 23 summer annual weed species. Weed Sci 62:267-279. doi.org/10.1614/WS-D-13-00116.1
Wortman SE, Davis AS, Schutte BJ, Lindquist JL, Cardina J, Felix J, Sprague CL, Dille JA, Ramirez AHM, Reicks G, Clay SA (2012) Local conditions, not regional gradients, drive demographic variation of giant ragweed (Ambrosia trifida) and common sunflower (Helianthus annuus) across northern U.S. maize belt. Weed Sci 60: 440-450. doi.org/10.1614/WS-D-11-00196.1
Wuerffel RJ, Young JM, Matthews JL, Davis VM, Johnson WG, Young BG (2015) Timing of soil-residual herbicide applications for control of giant ragweed (Ambrosia trifida) Weed Technol 29:771-781. doi.org/10.1614/WT-D-15-00018.1